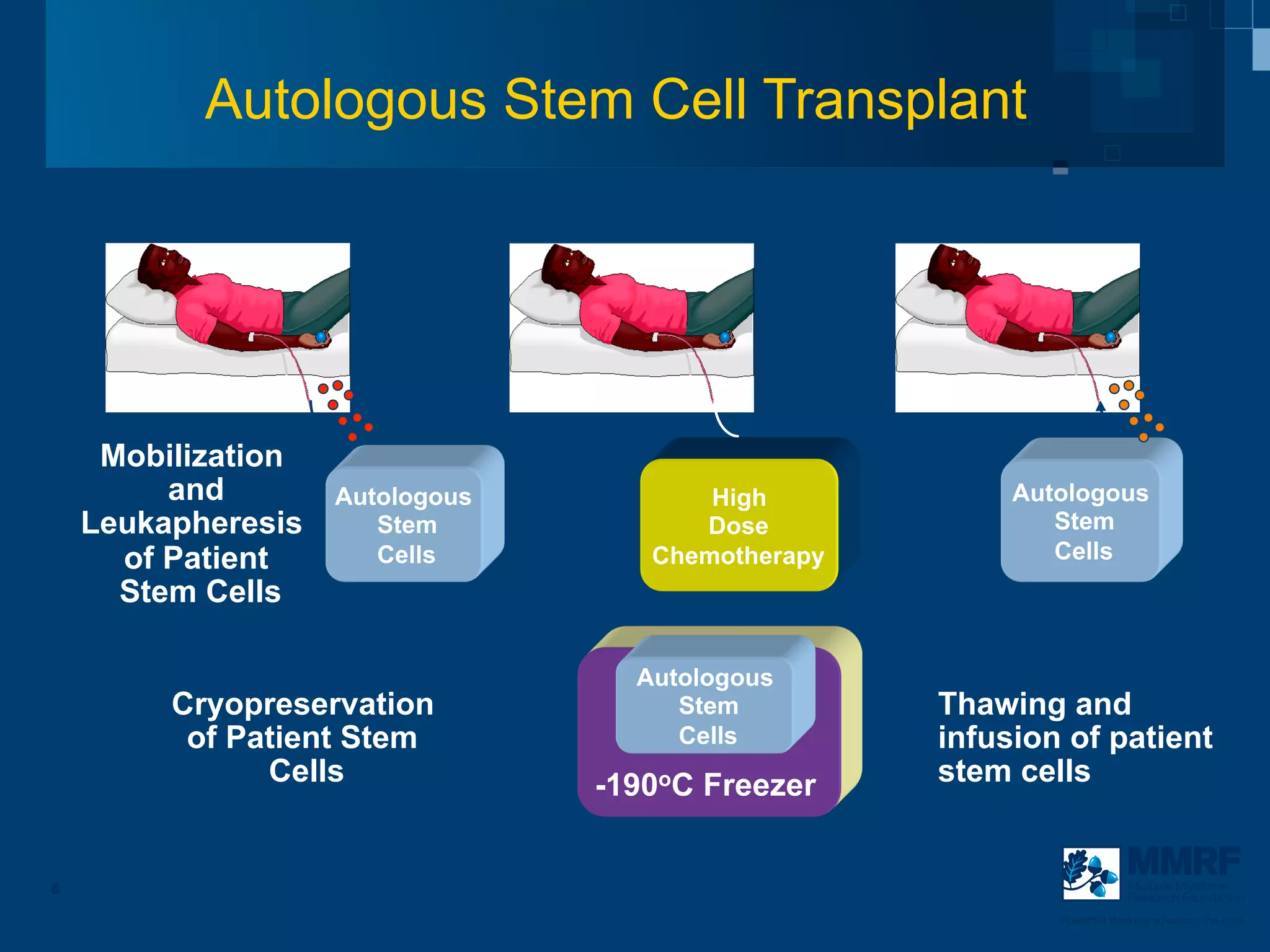
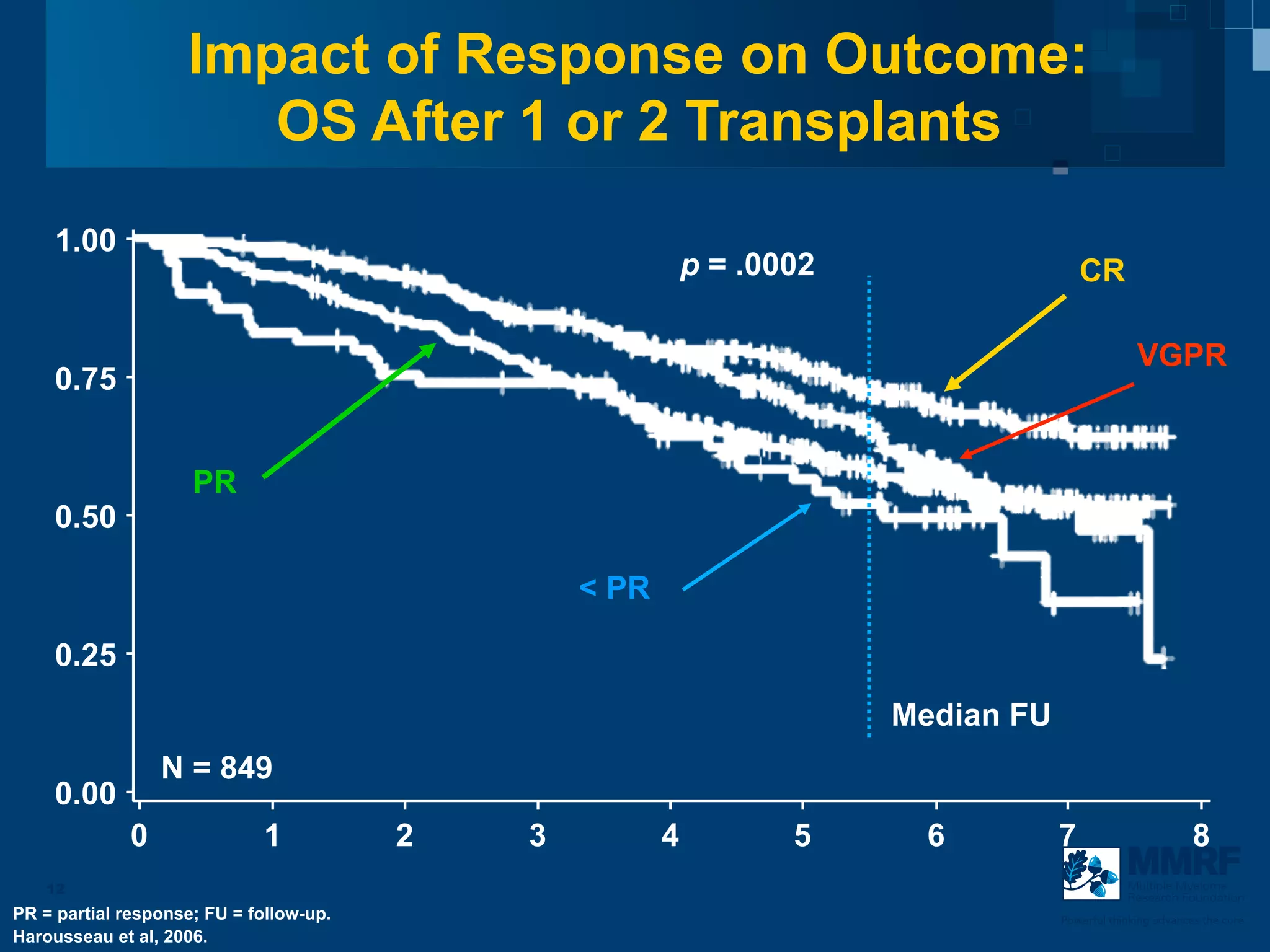
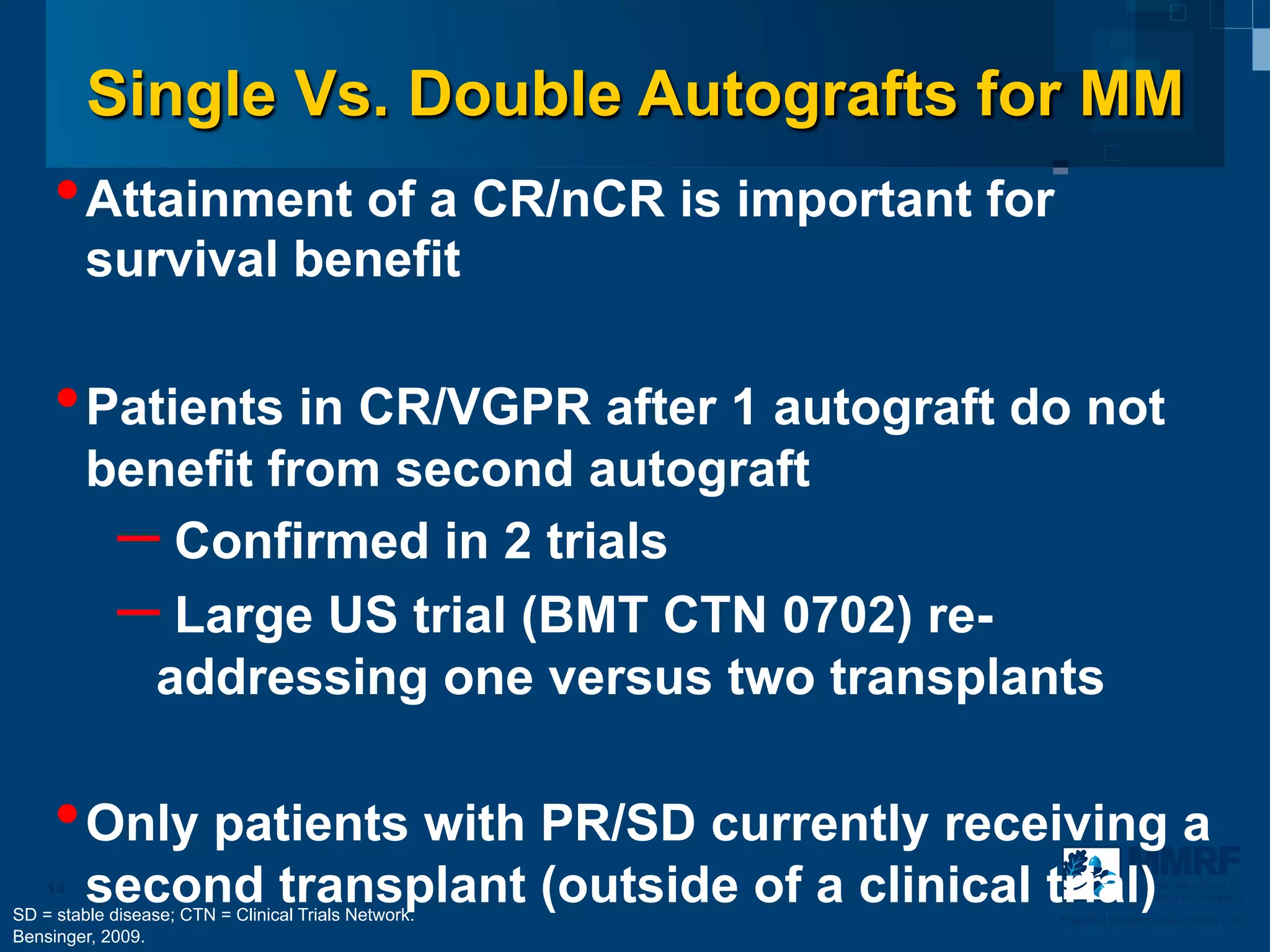
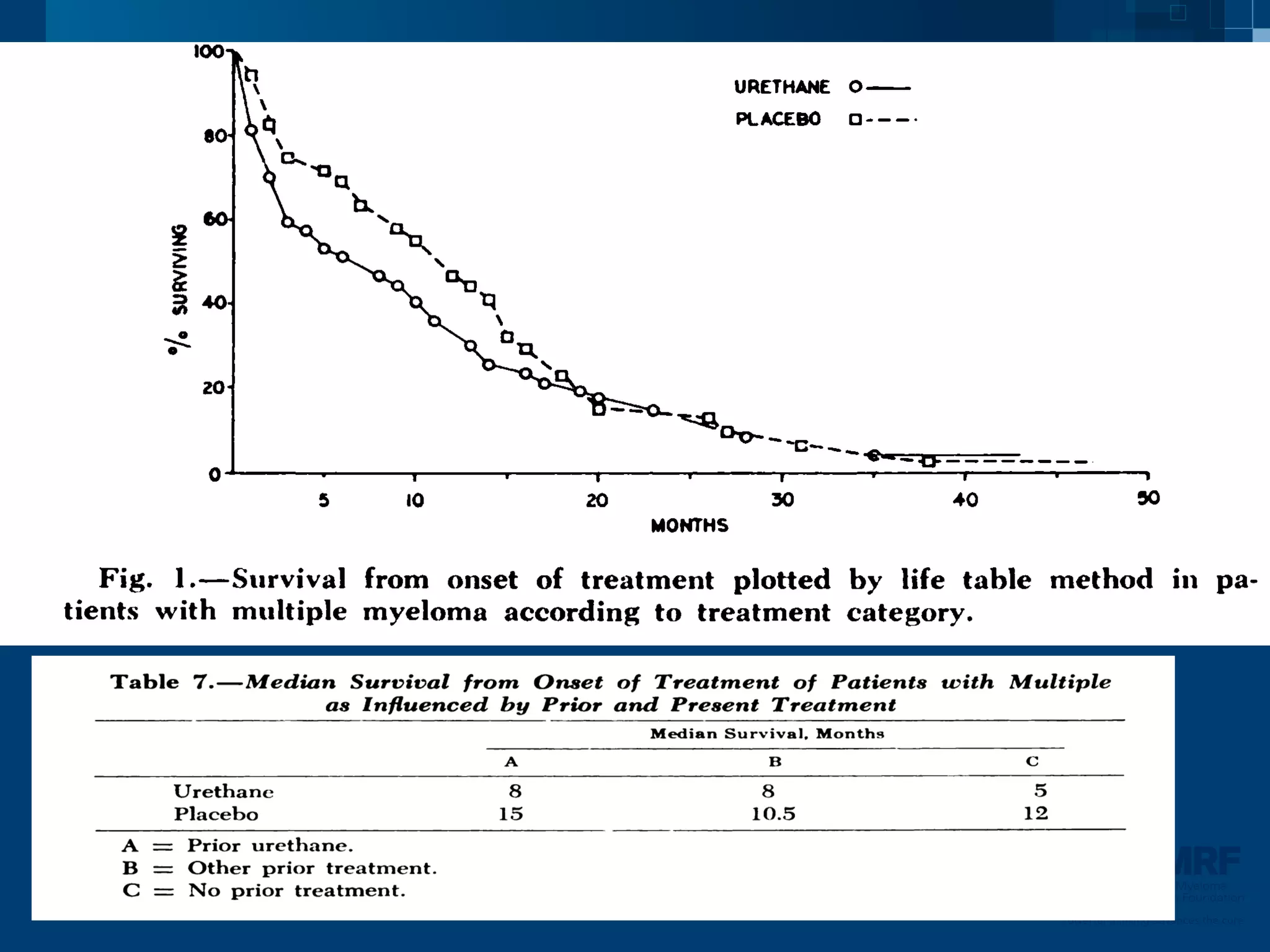
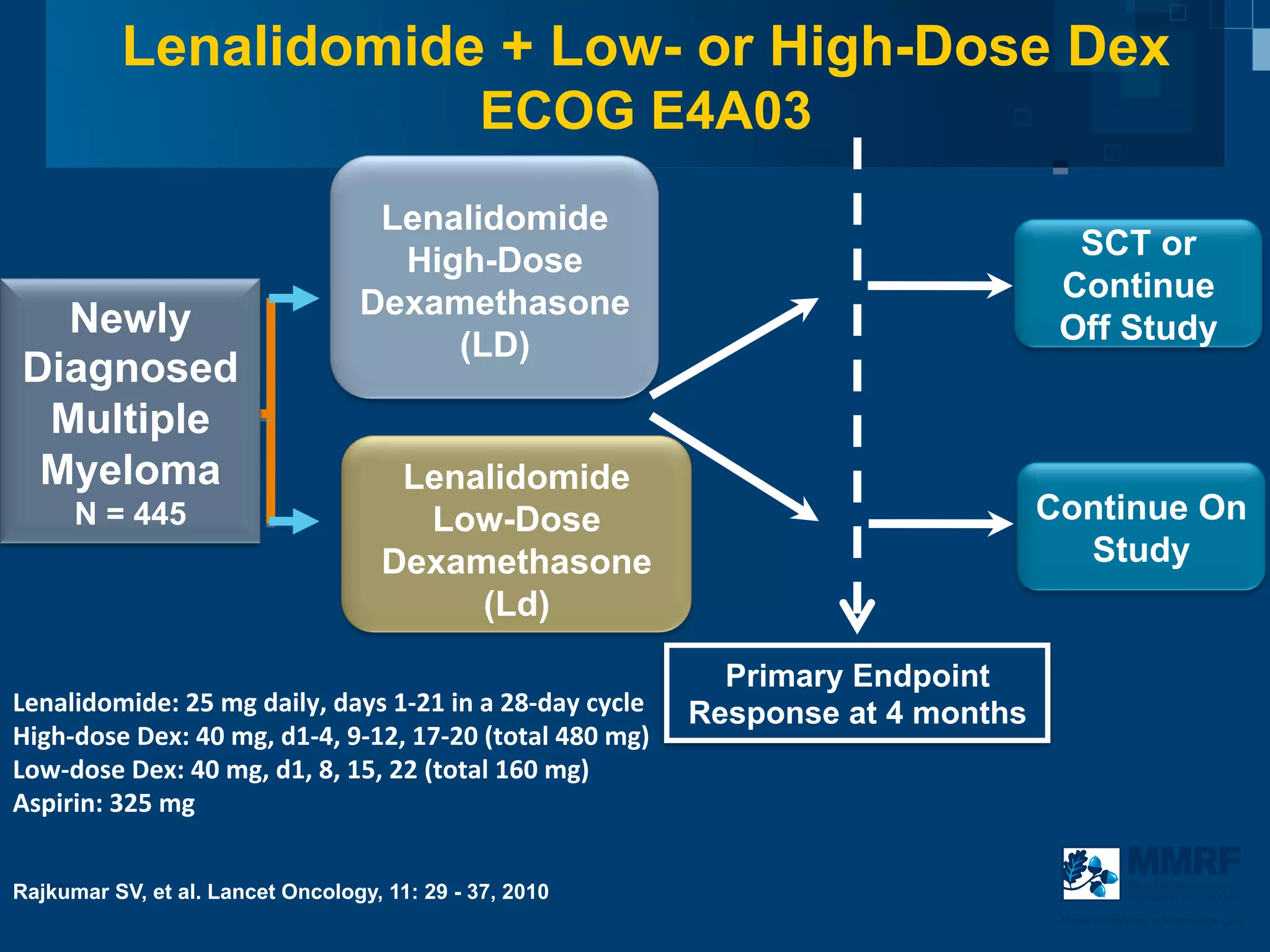
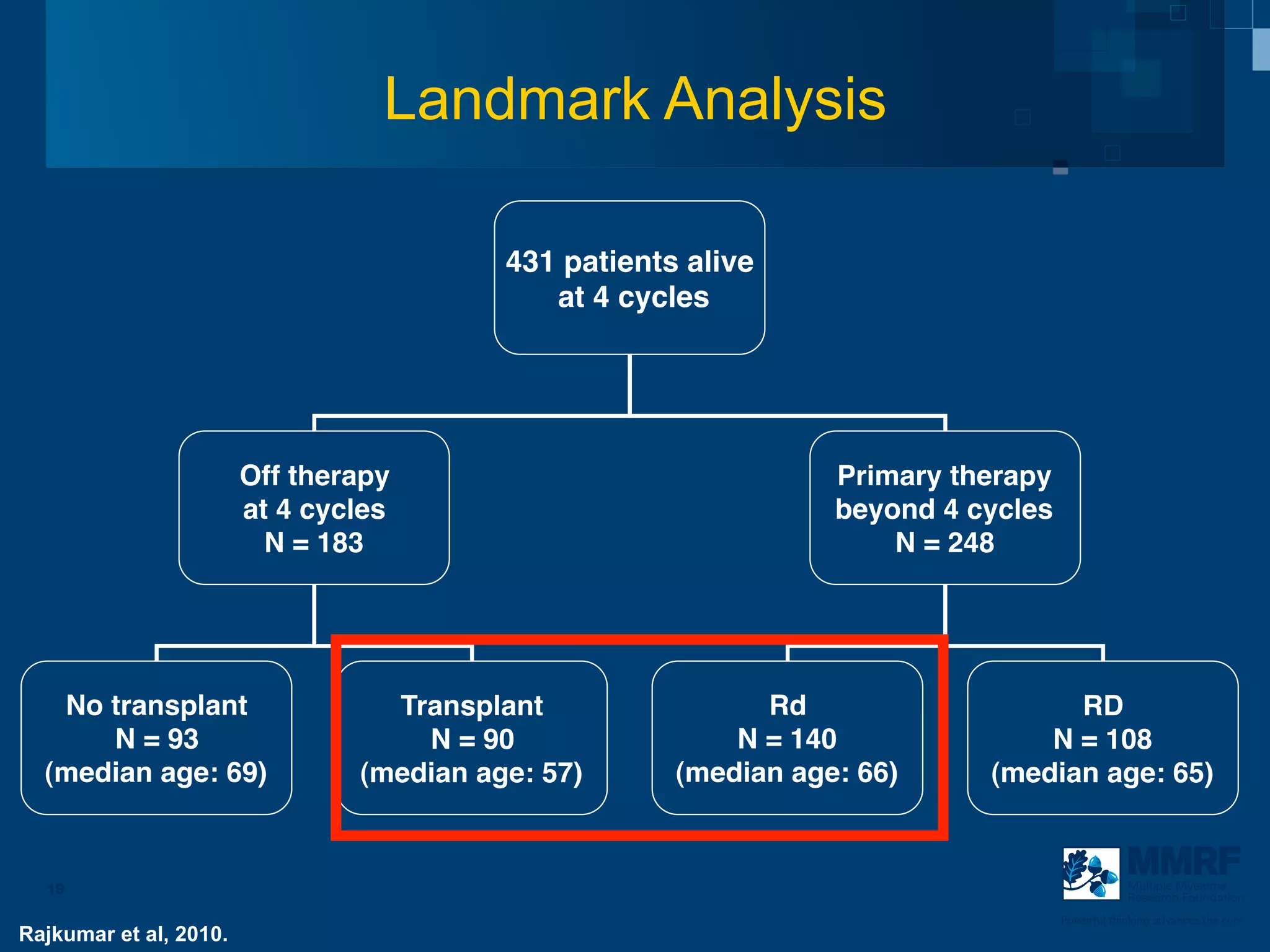
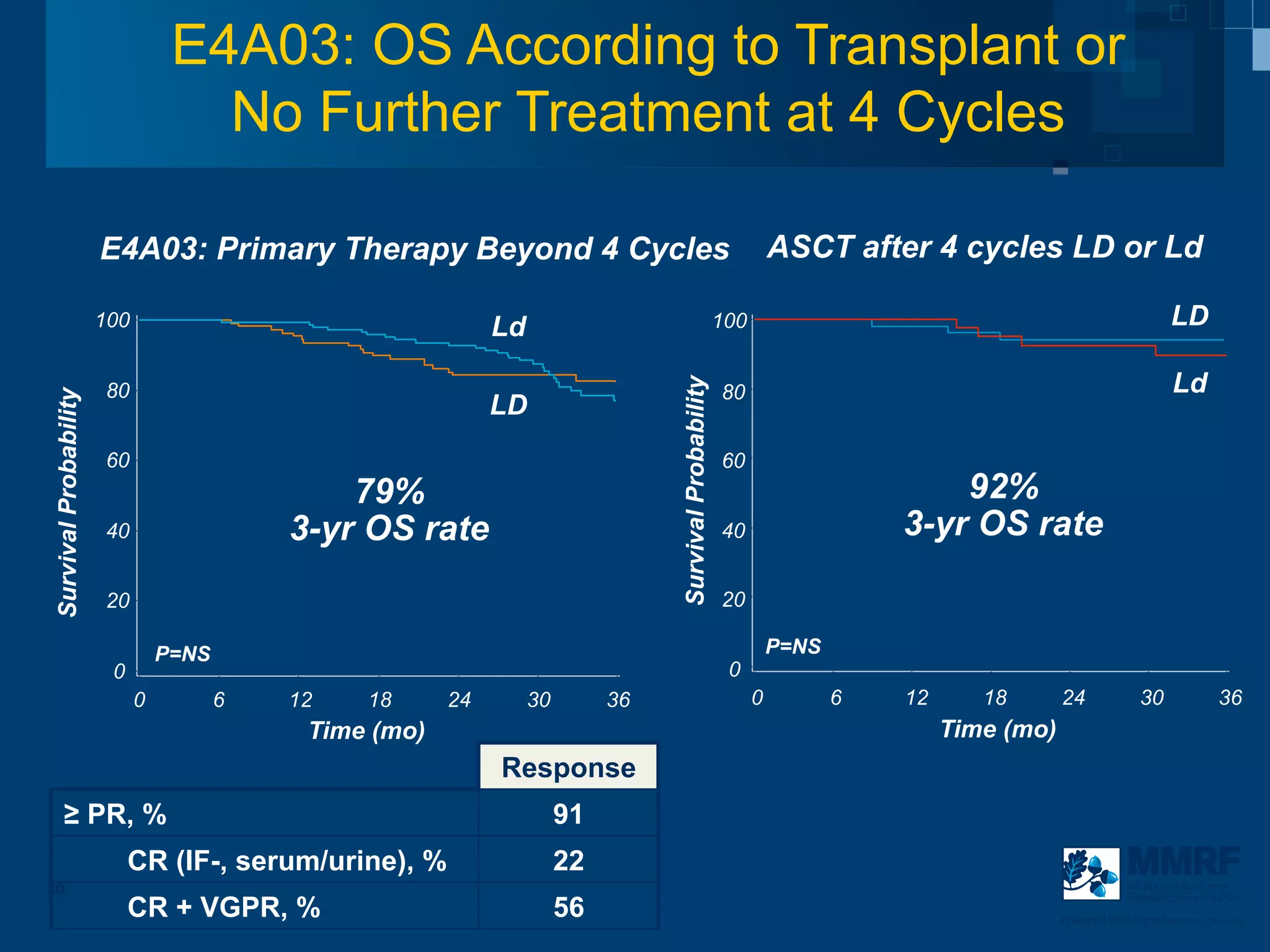
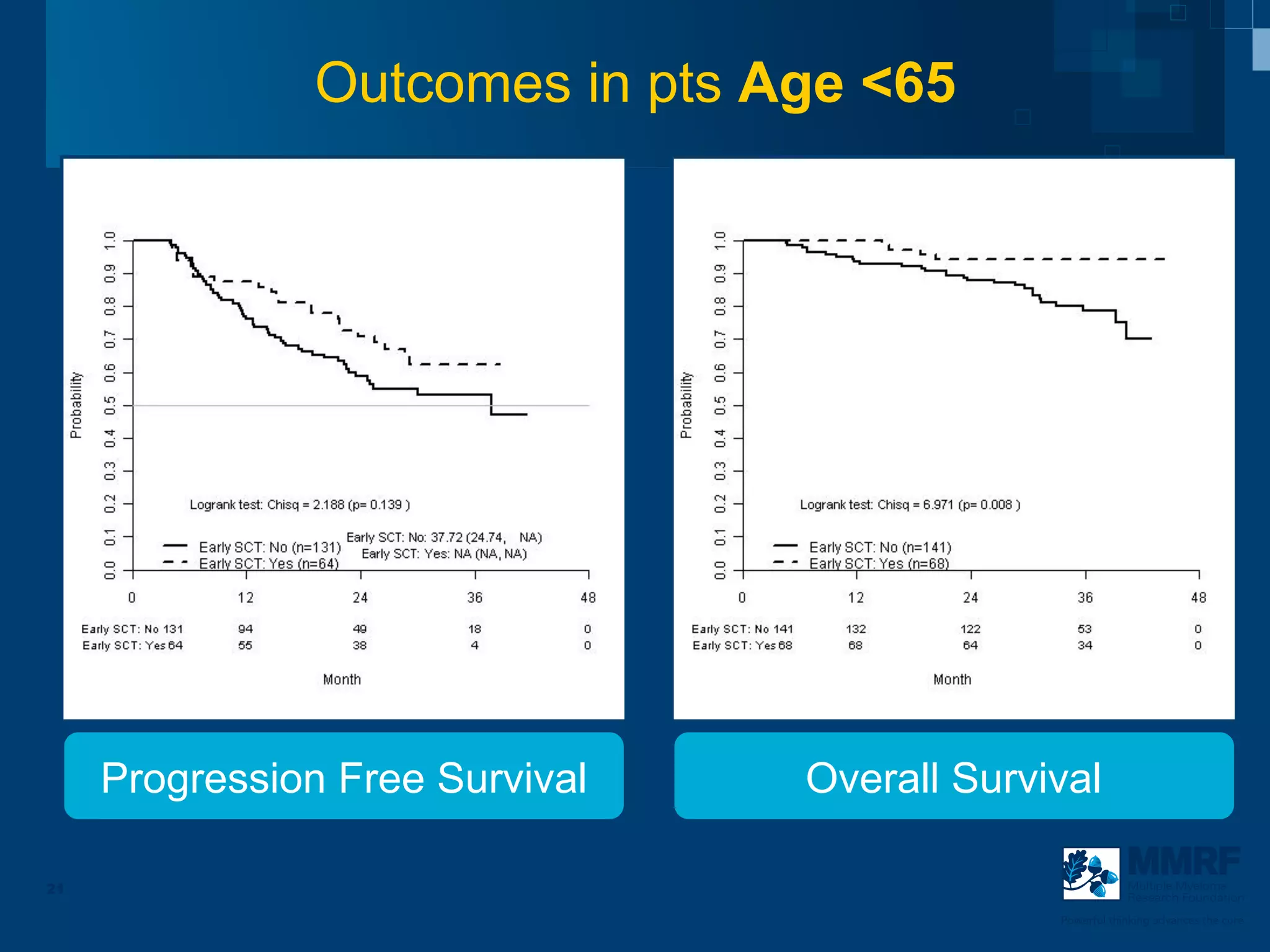
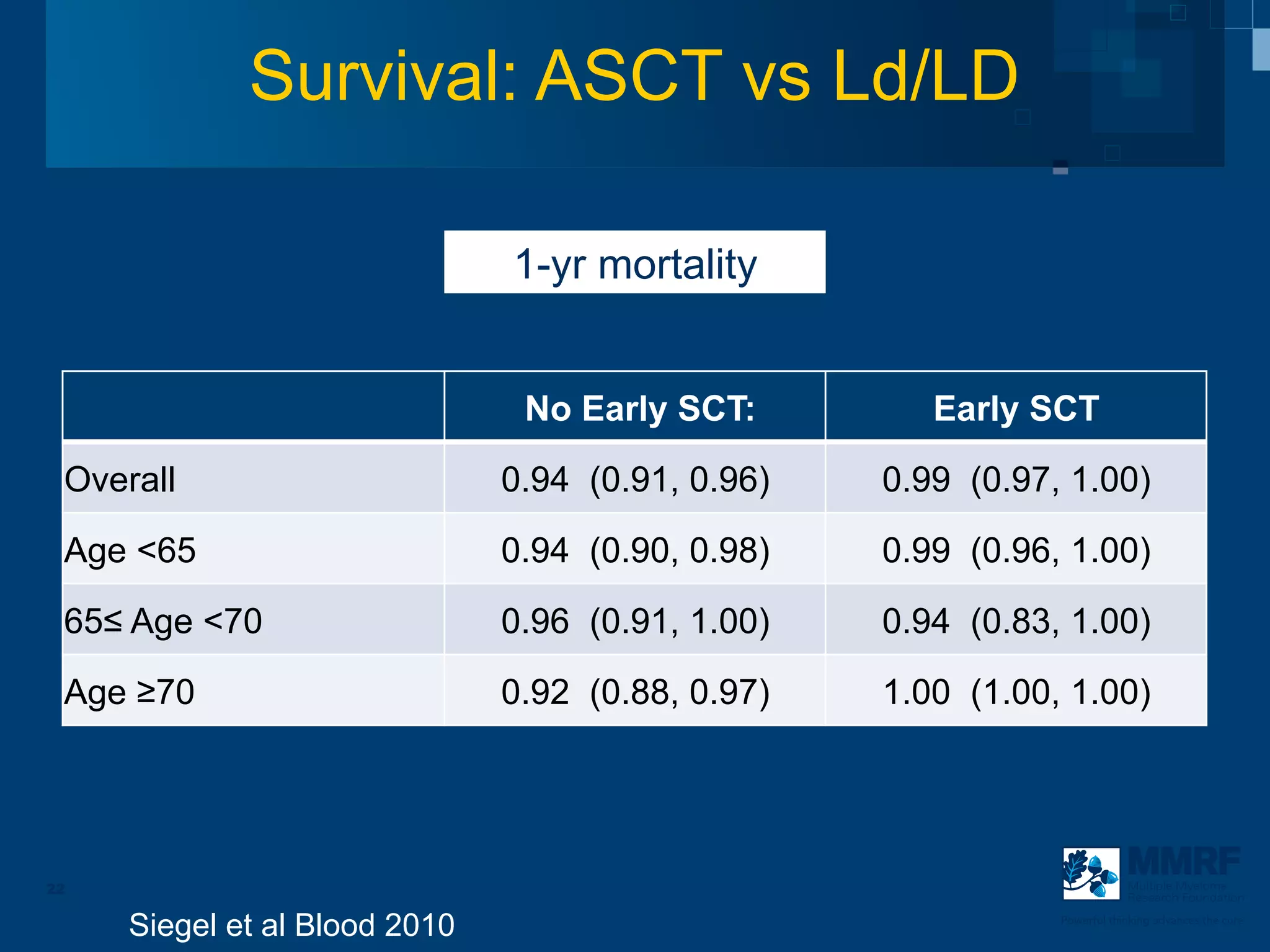
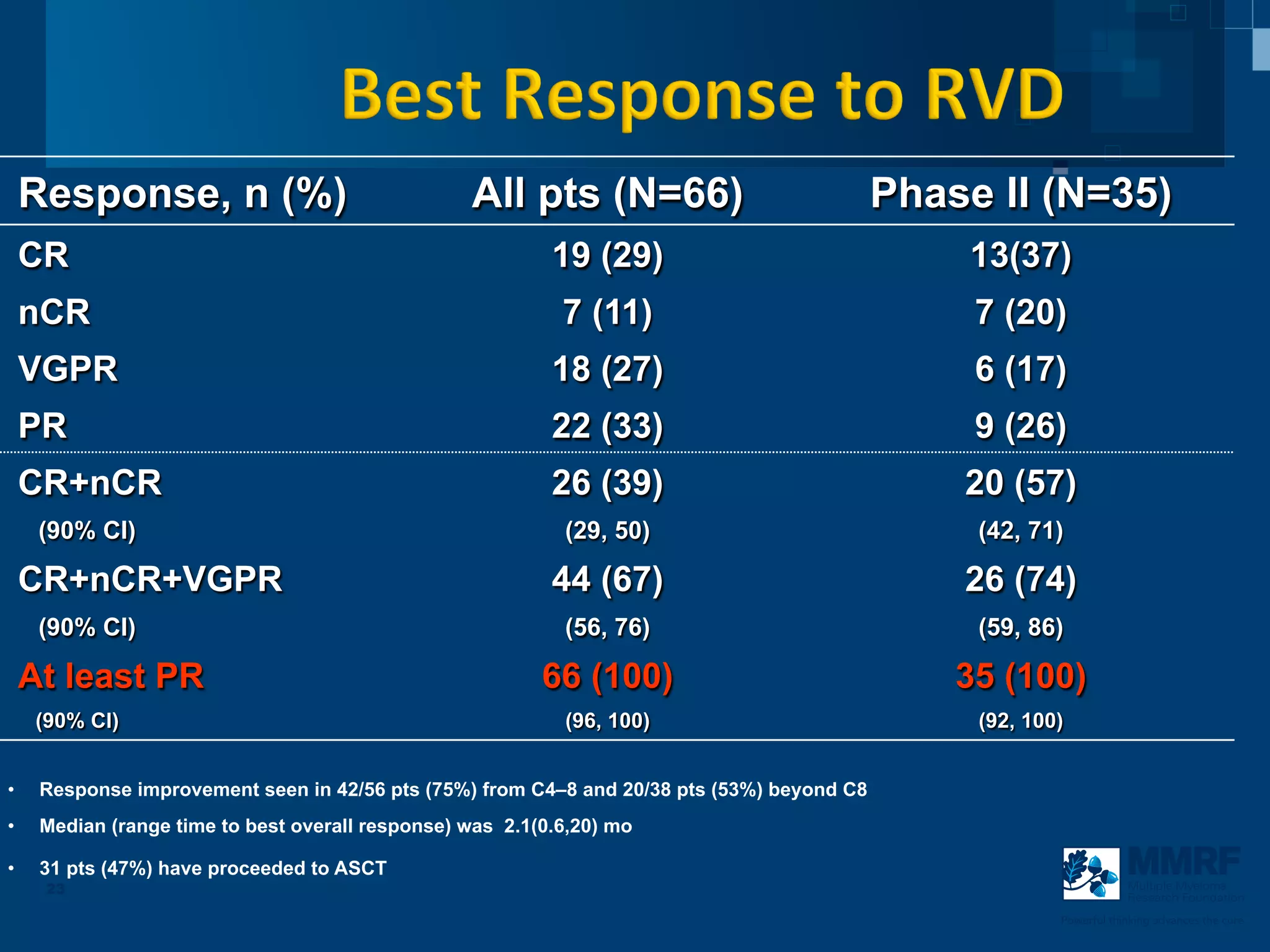
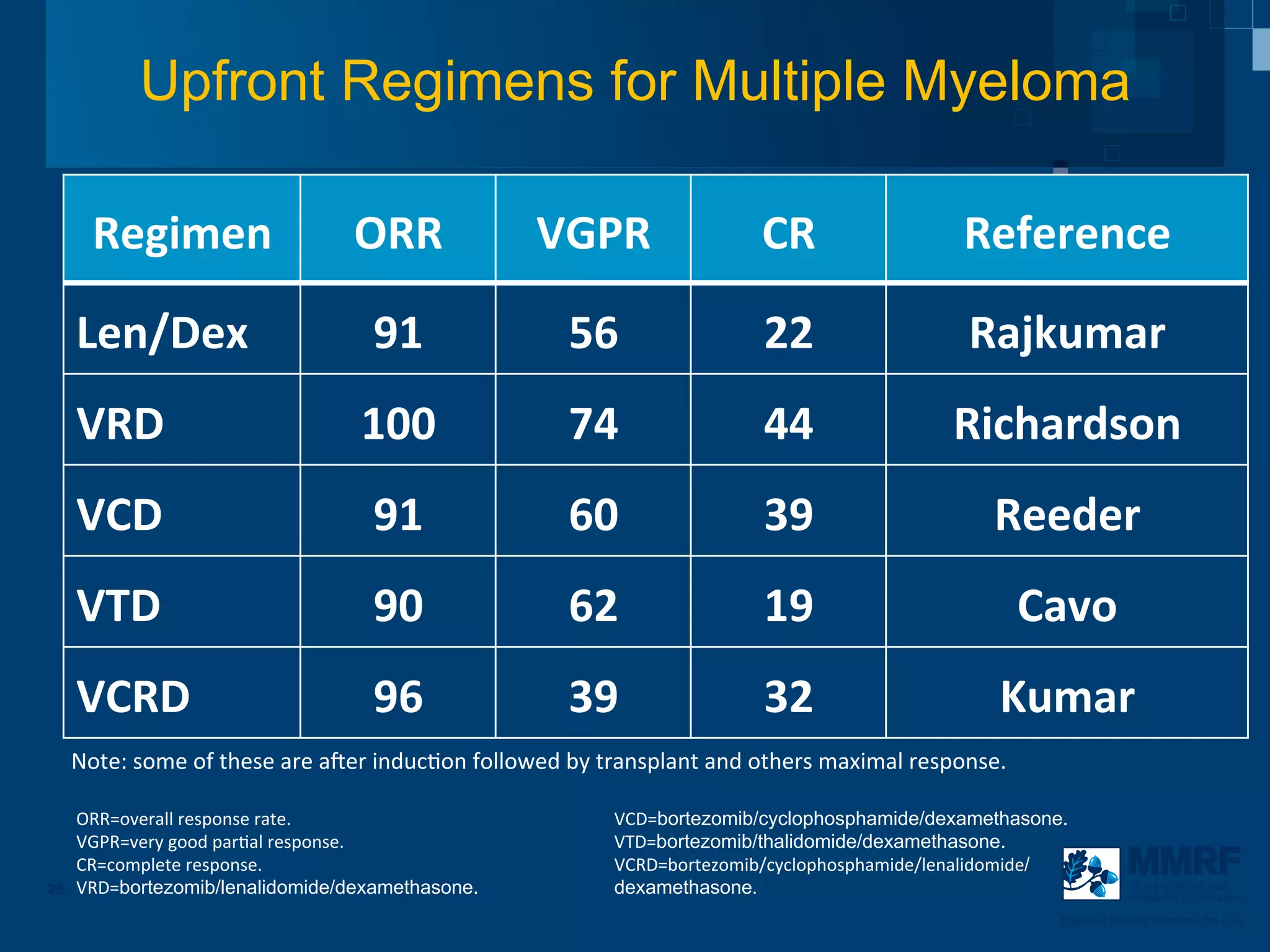
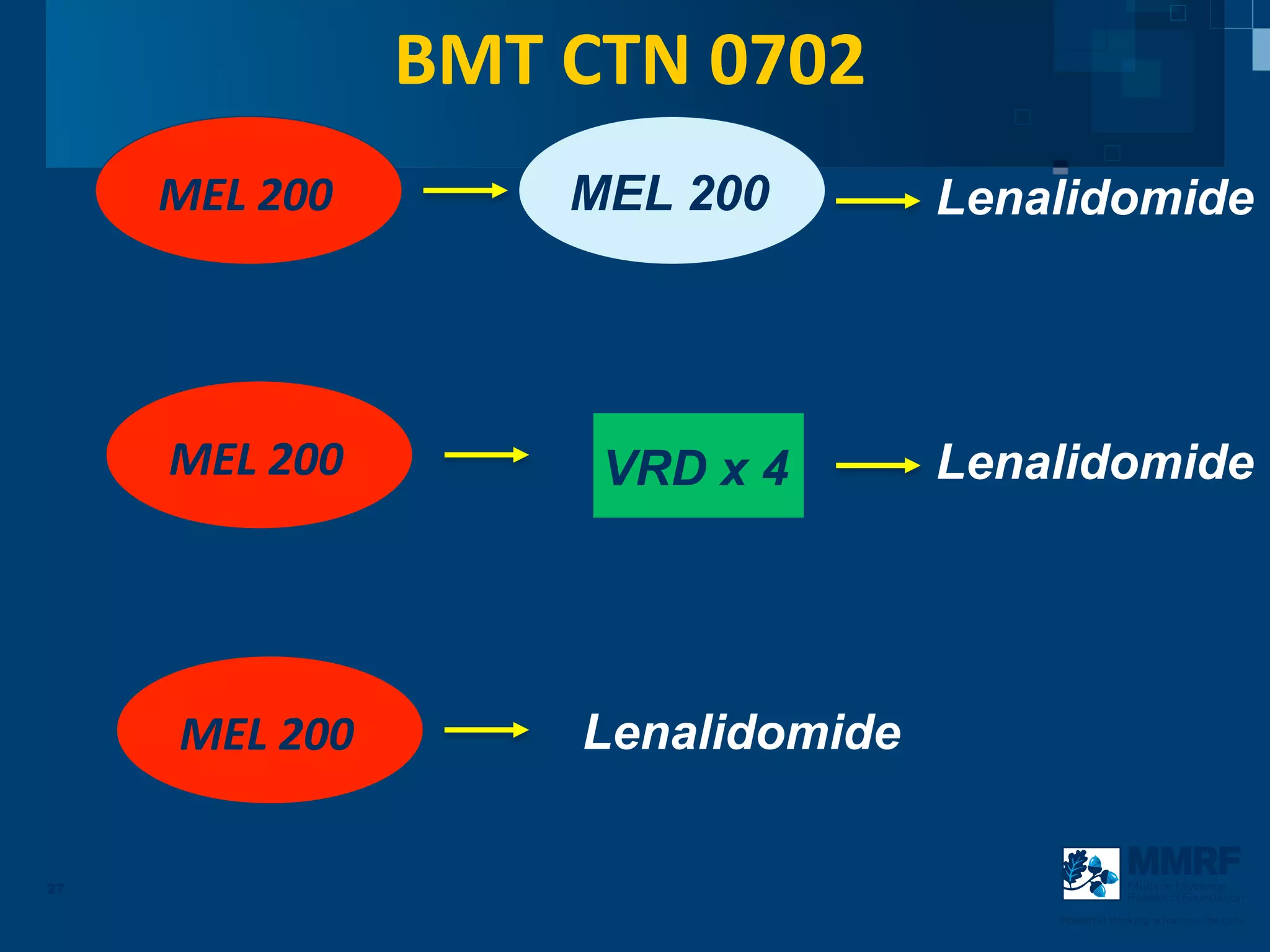
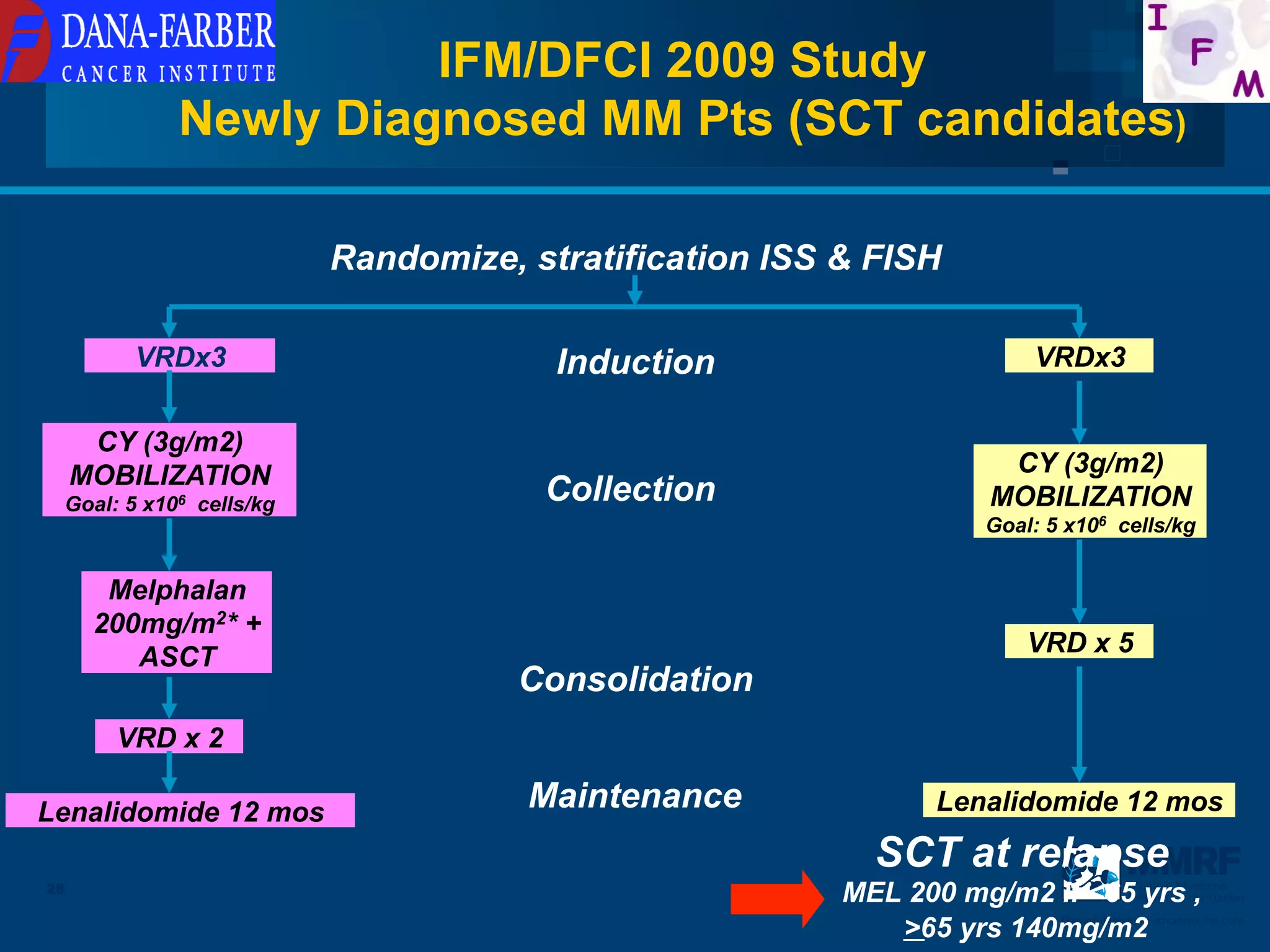
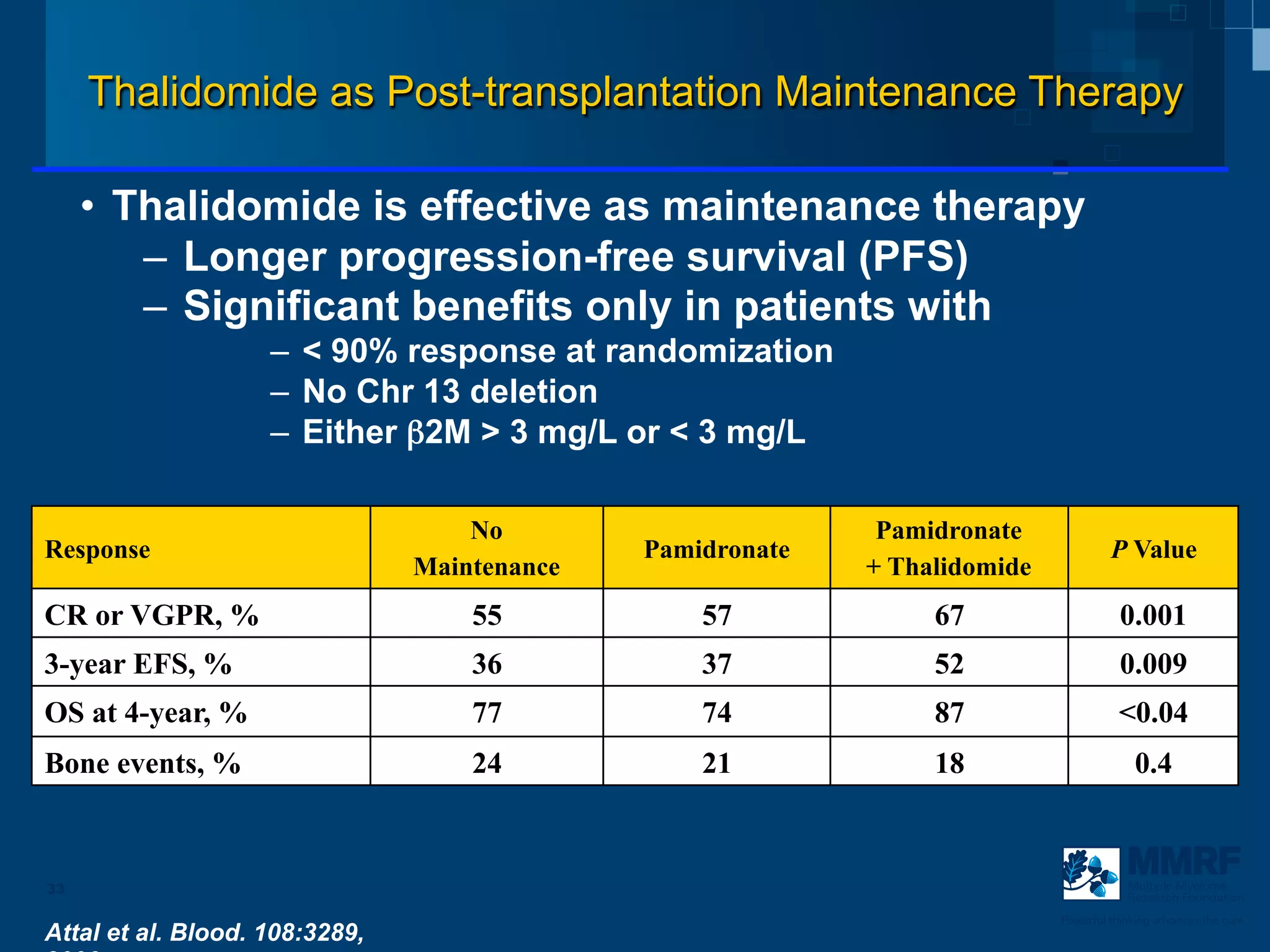
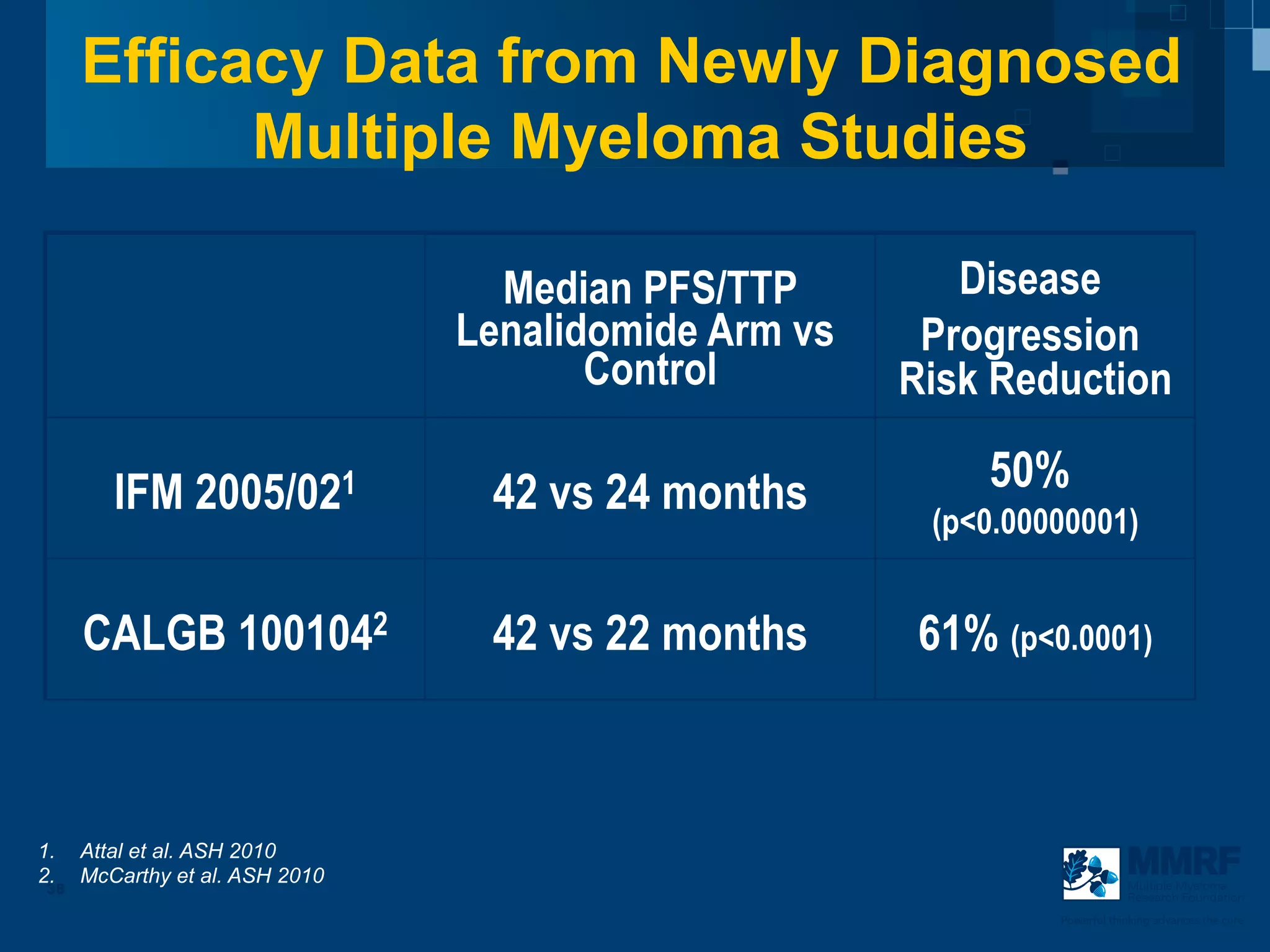
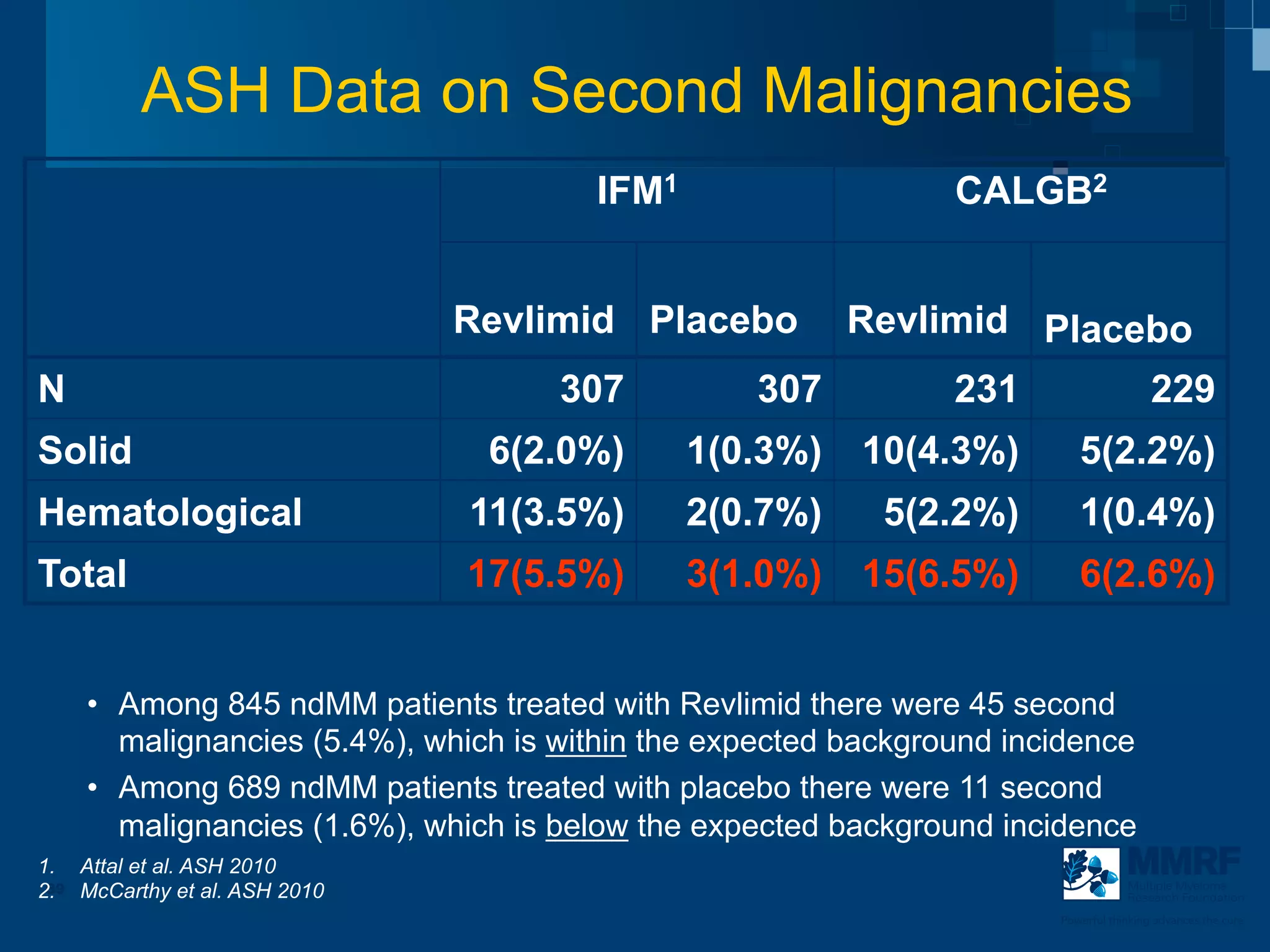
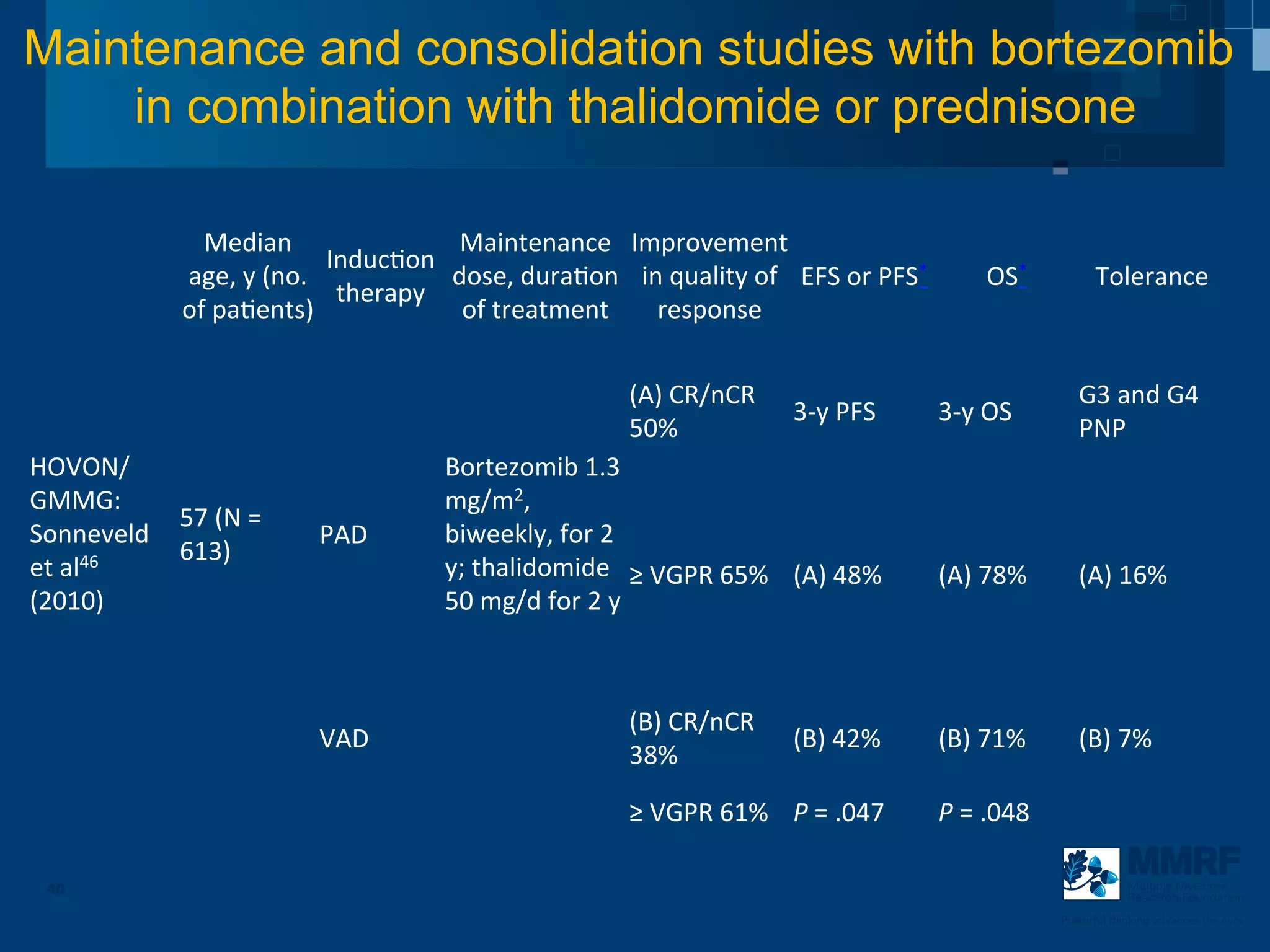
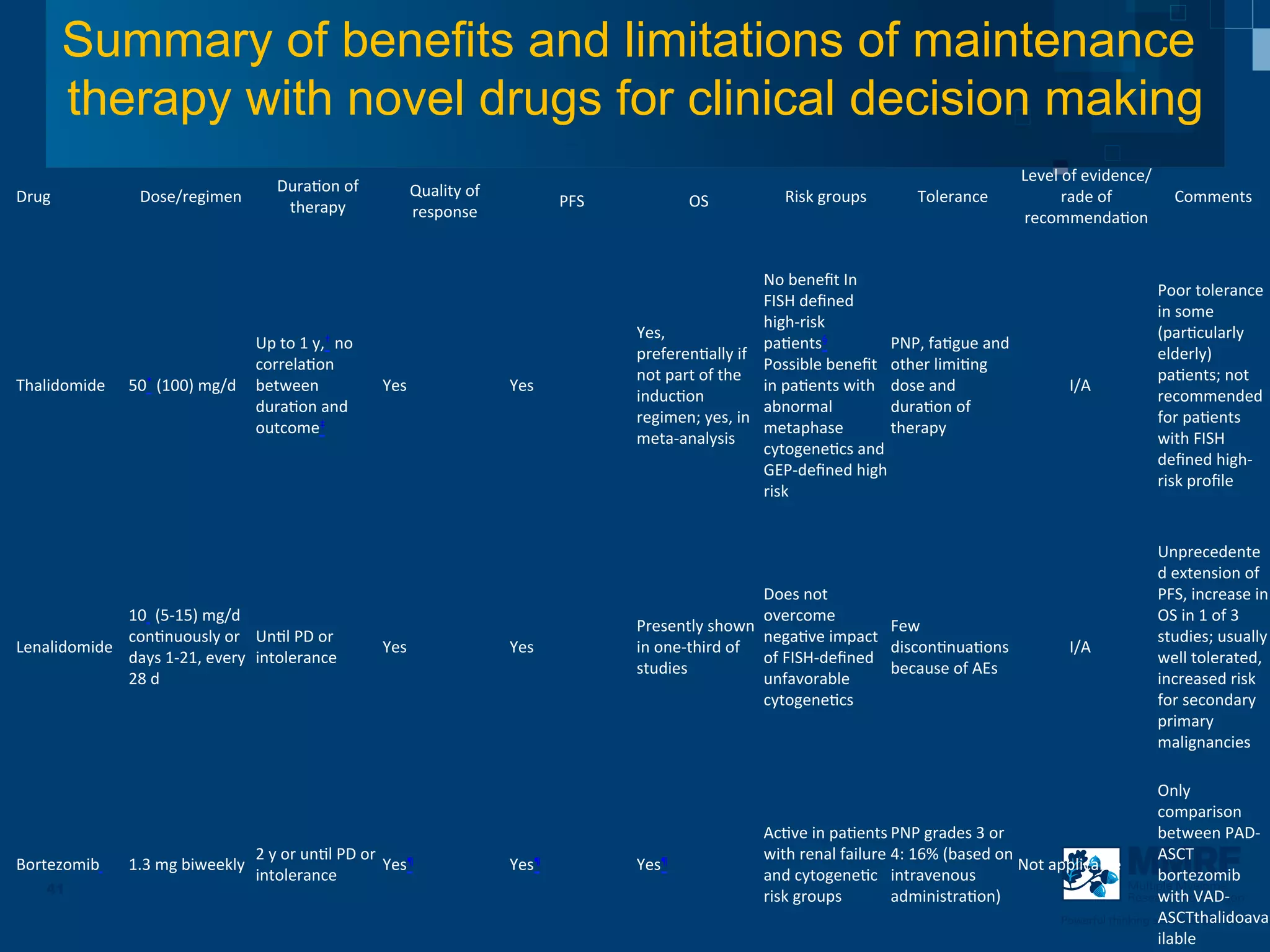
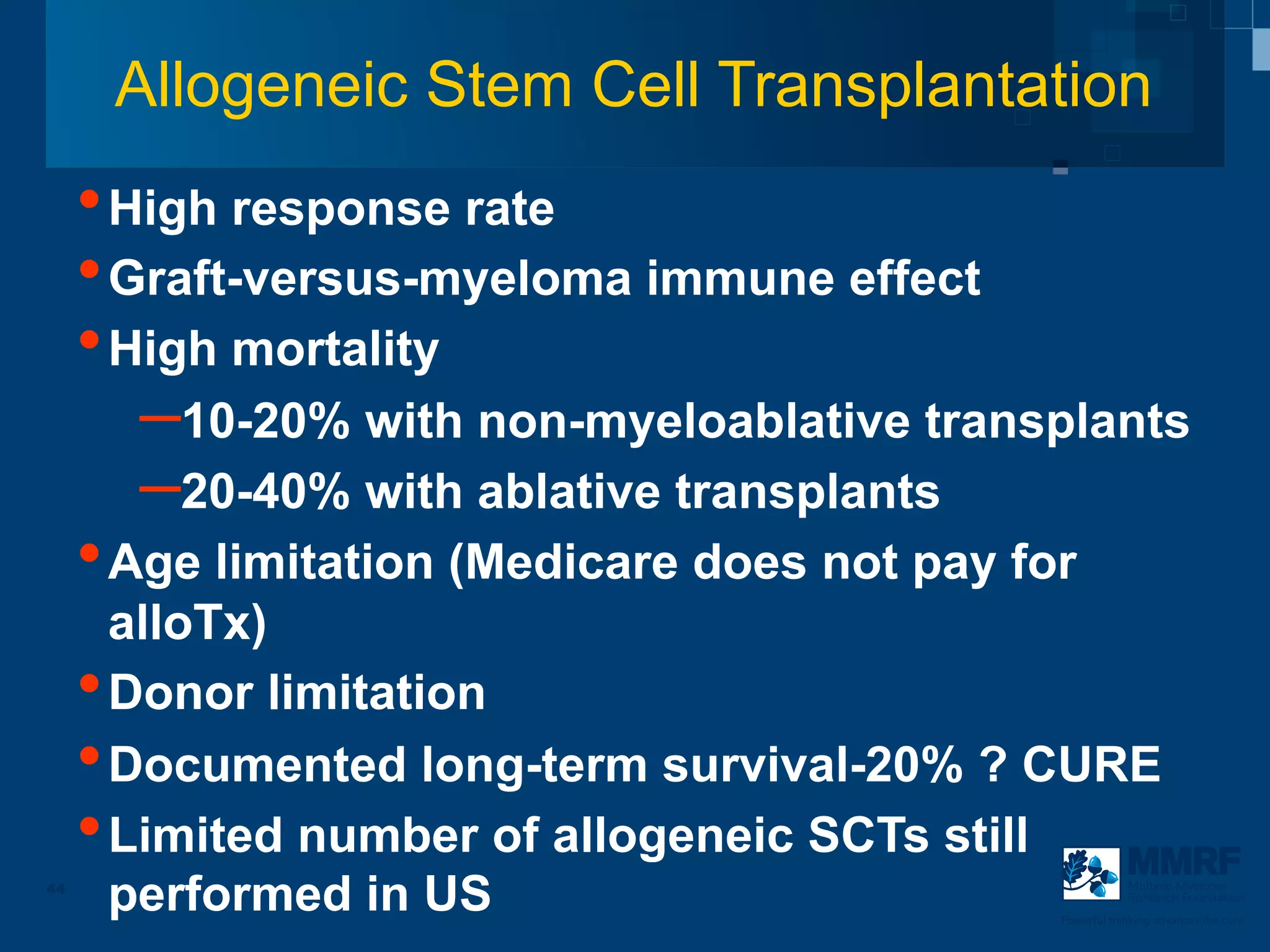
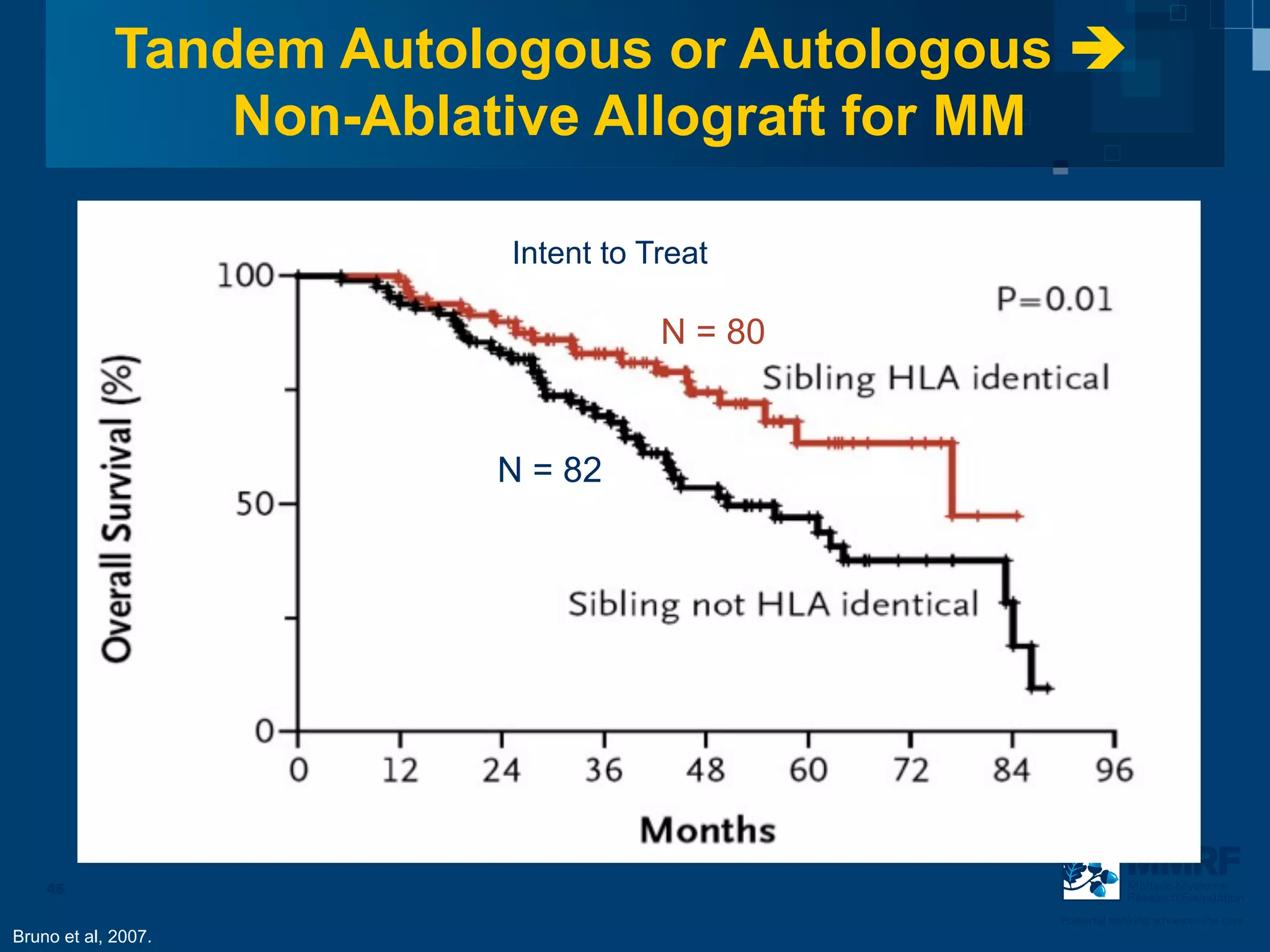
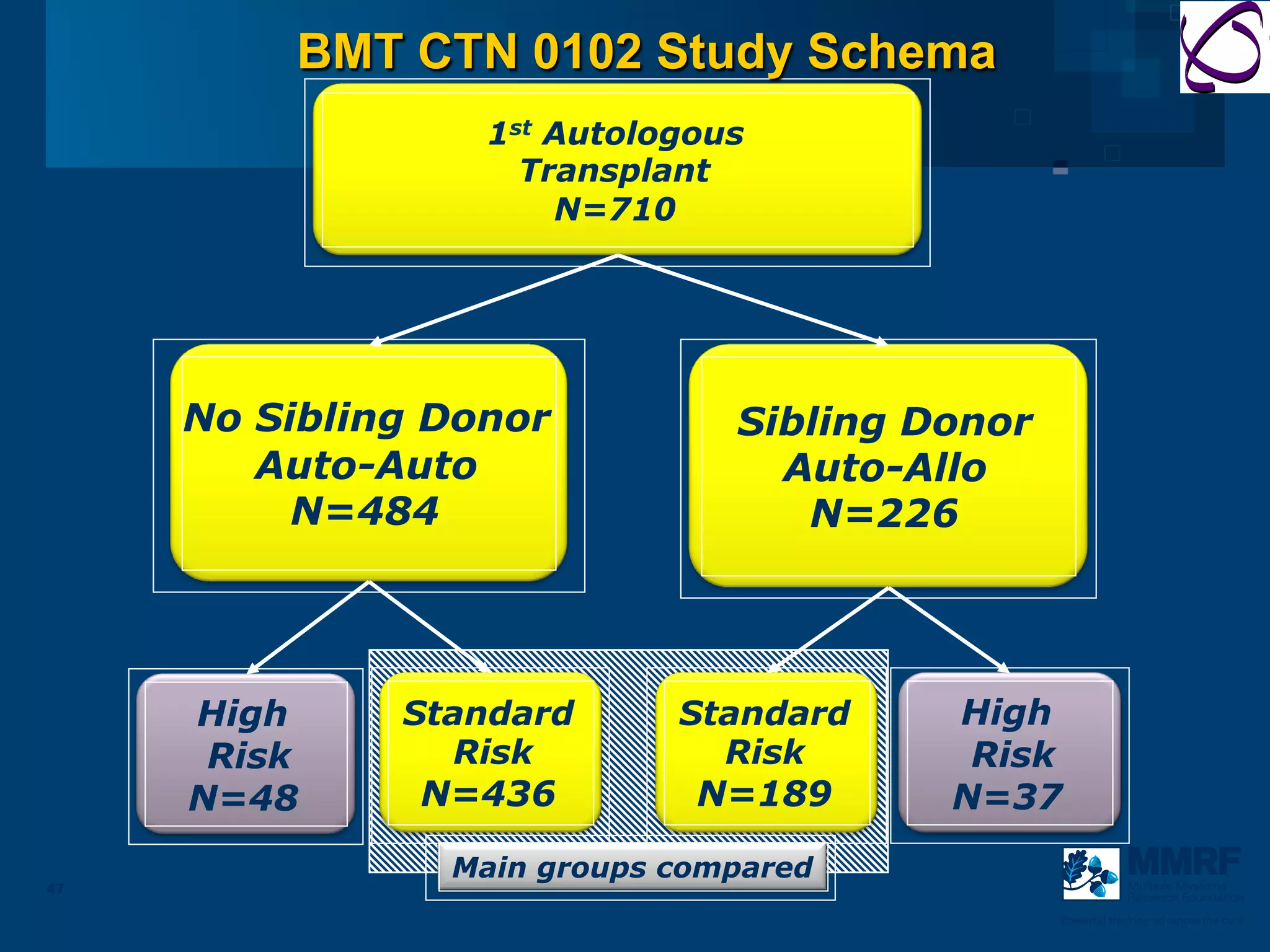
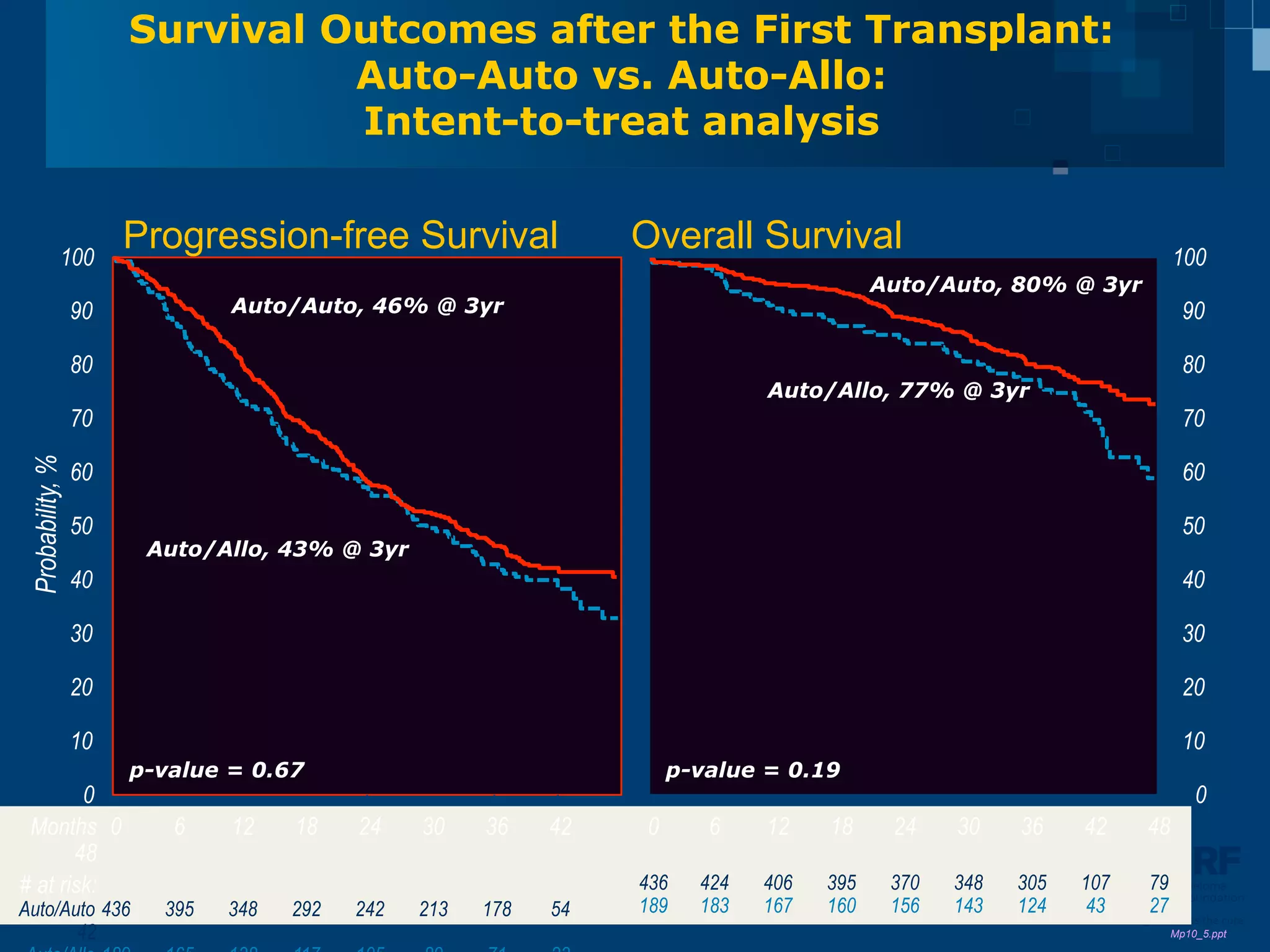
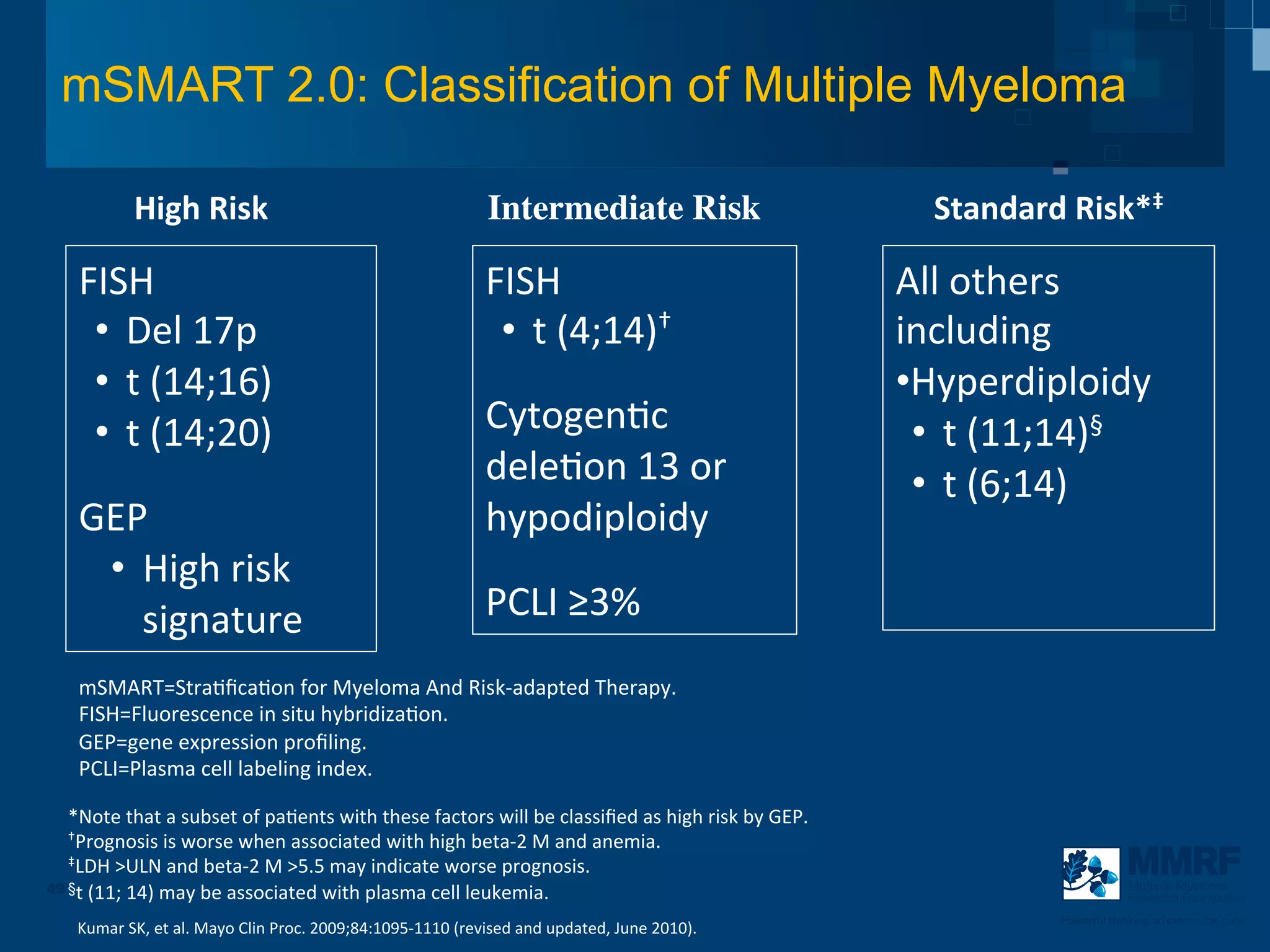
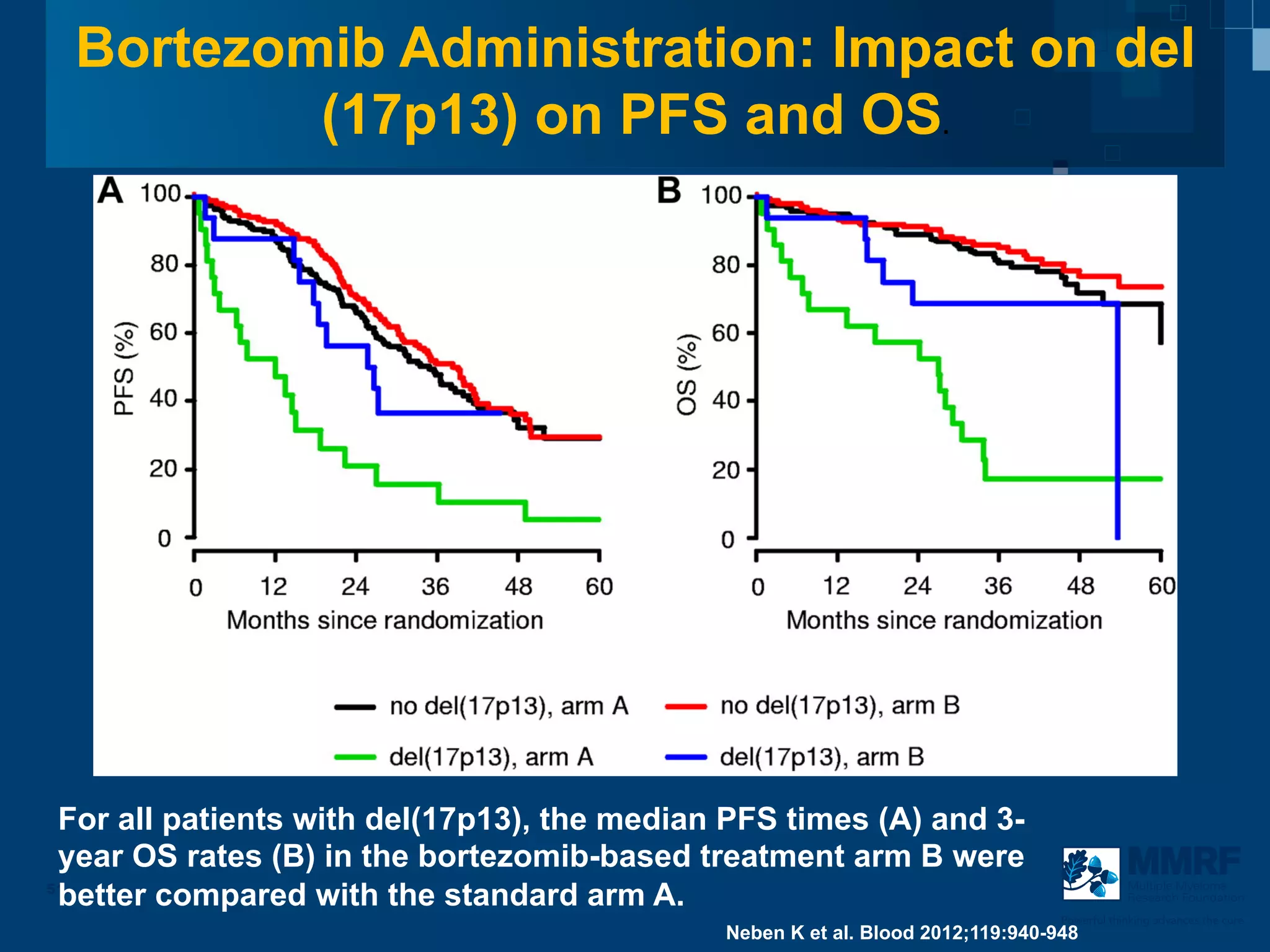
The document discusses stem cell transplantation as a treatment for multiple myeloma, emphasizing the effectiveness of high-dose chemotherapy and the use of autologous or allogeneic stem cells. It outlines the rationale, procedures, and outcomes related to autologous stem cell transplants, including survival benefits and the importance of response rates. The document also covers the role of maintenance therapy with novel agents to improve long-term outcomes in patients post-transplant.