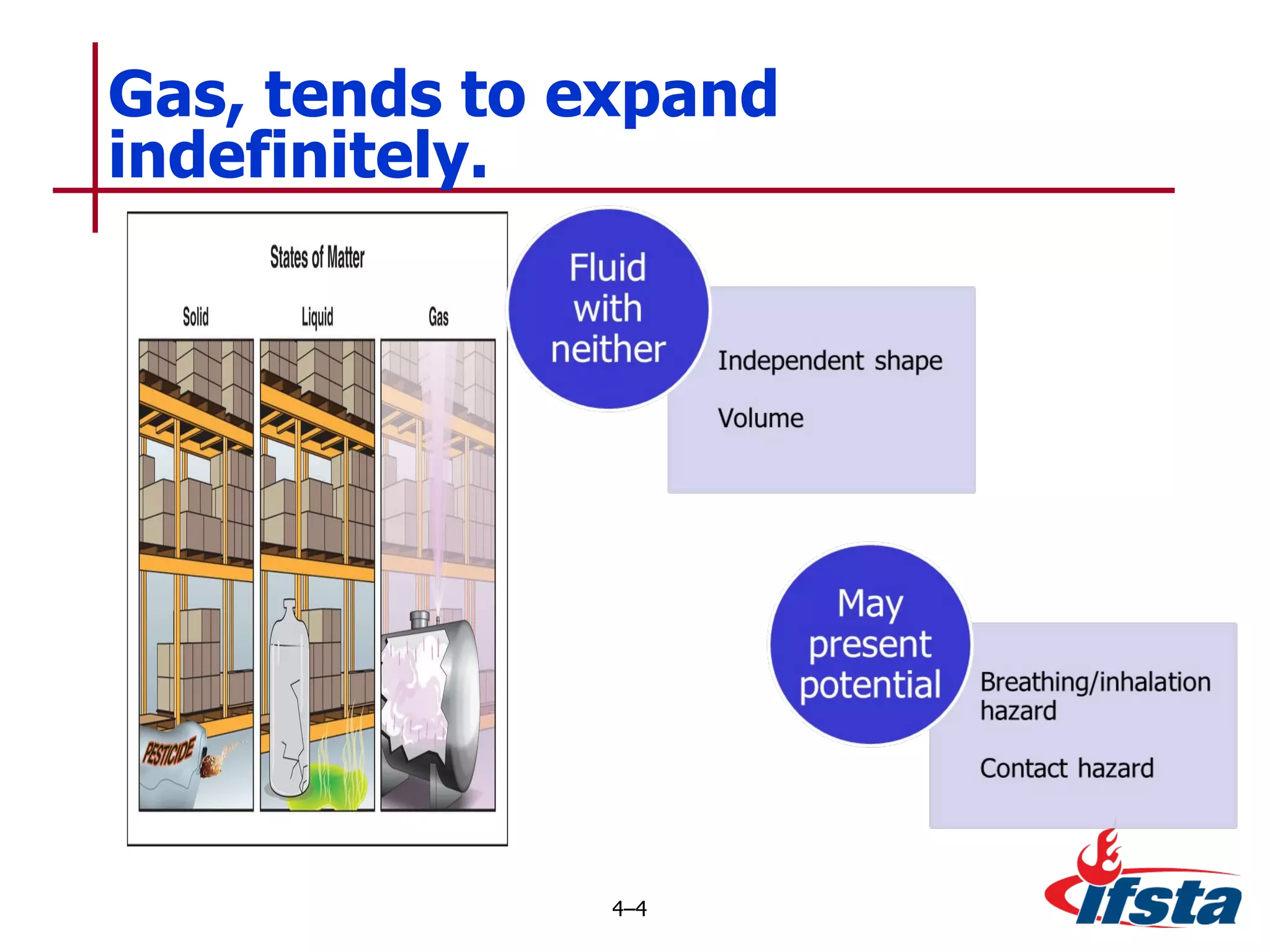
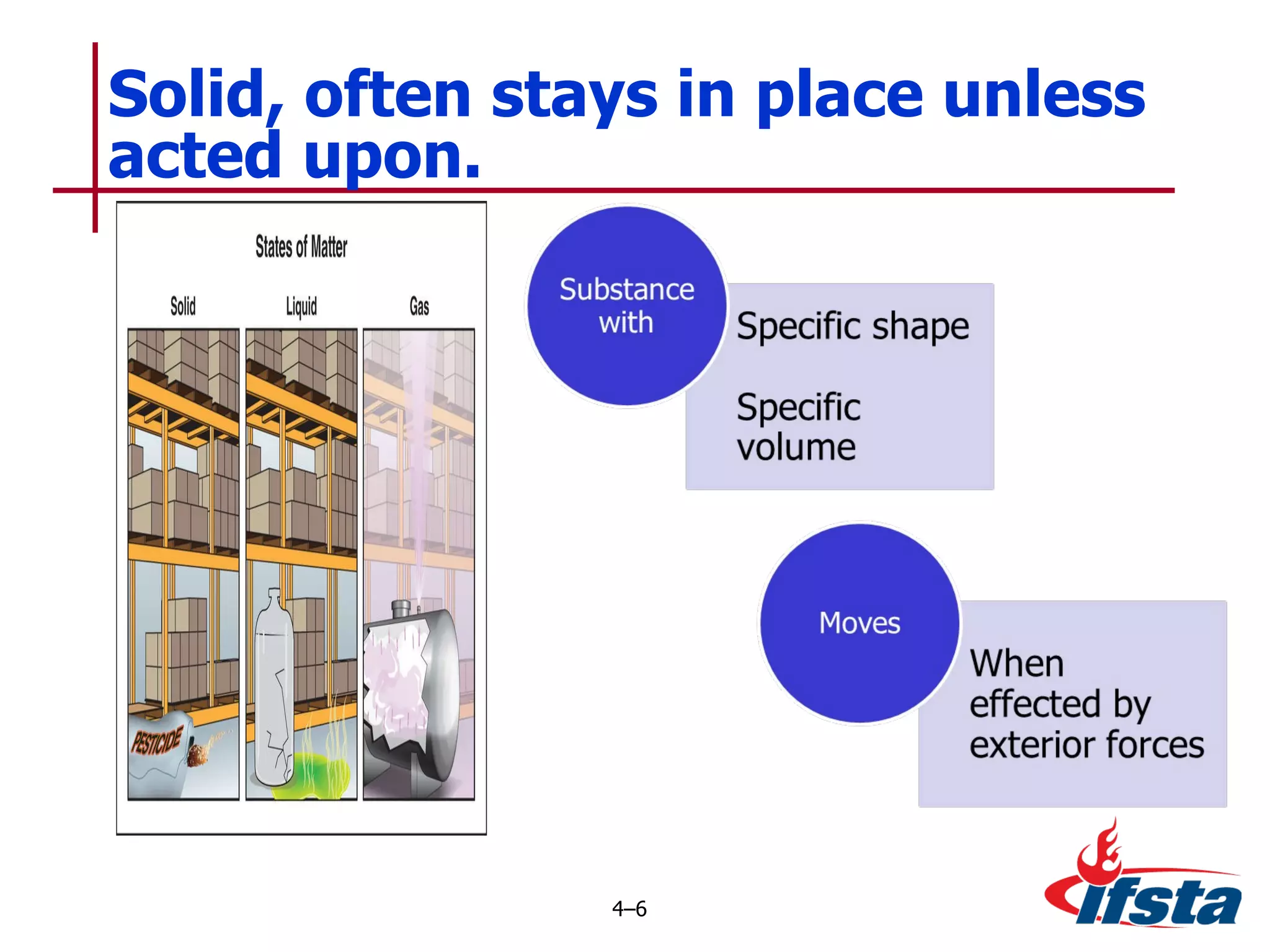
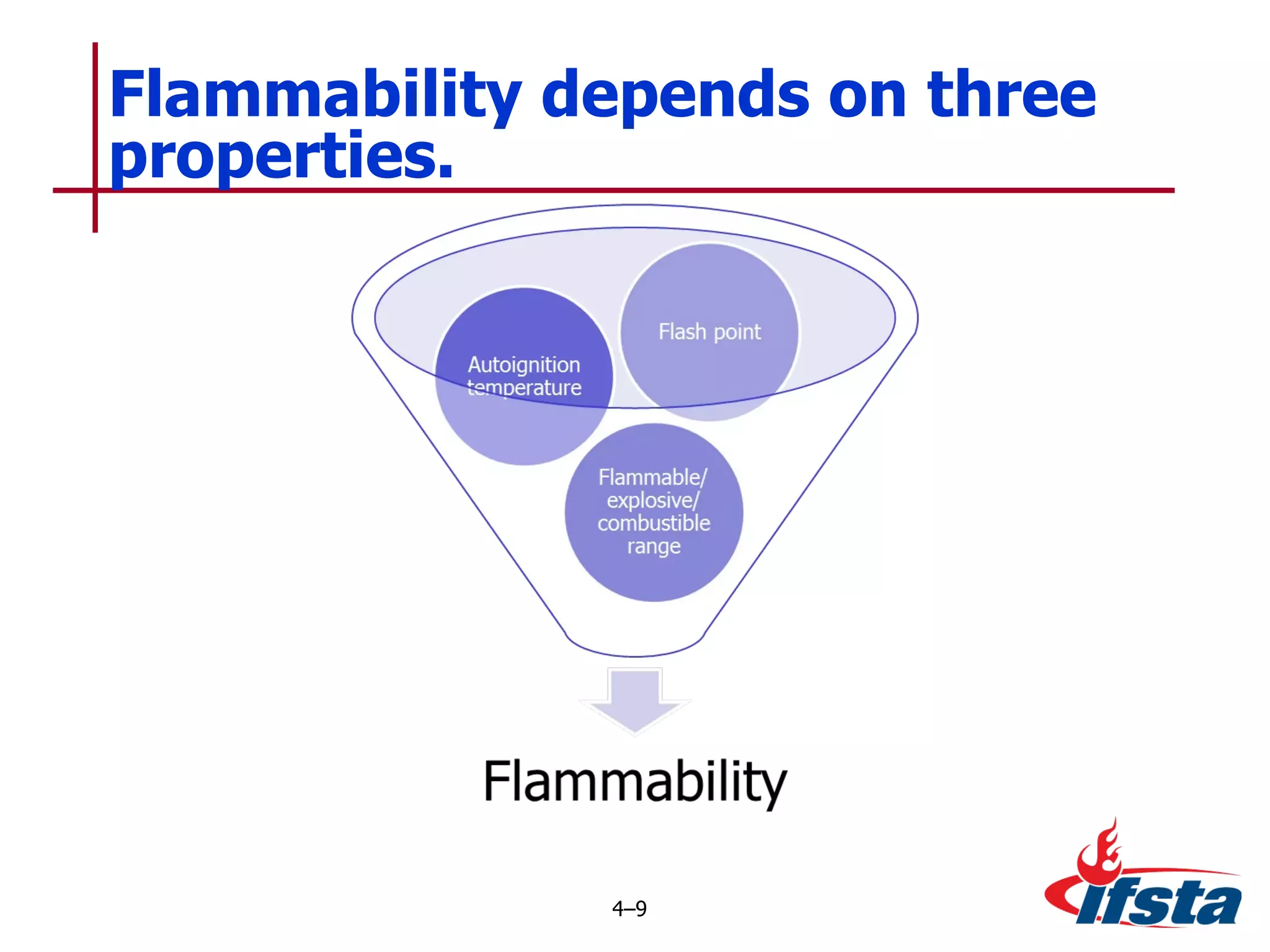
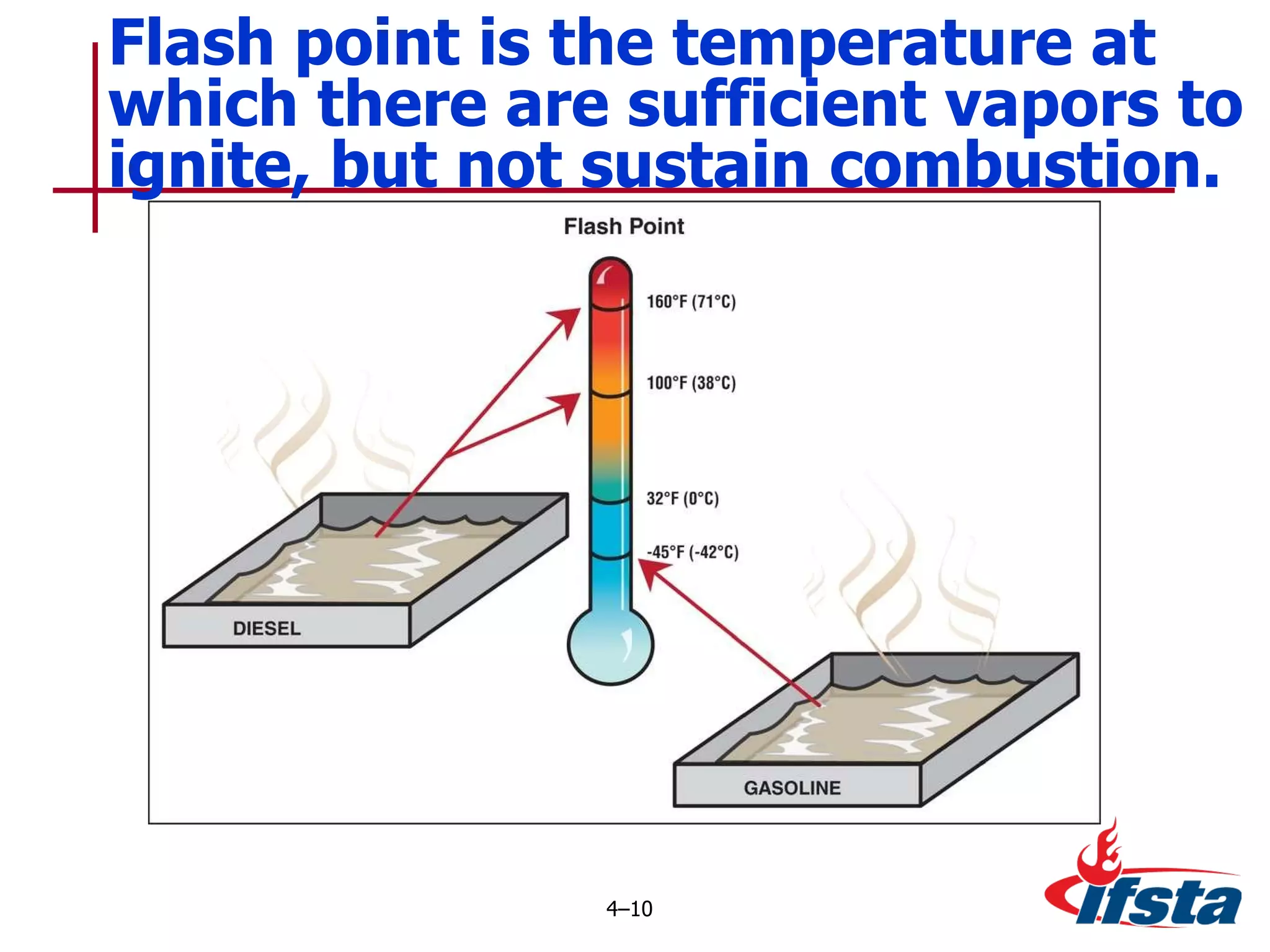
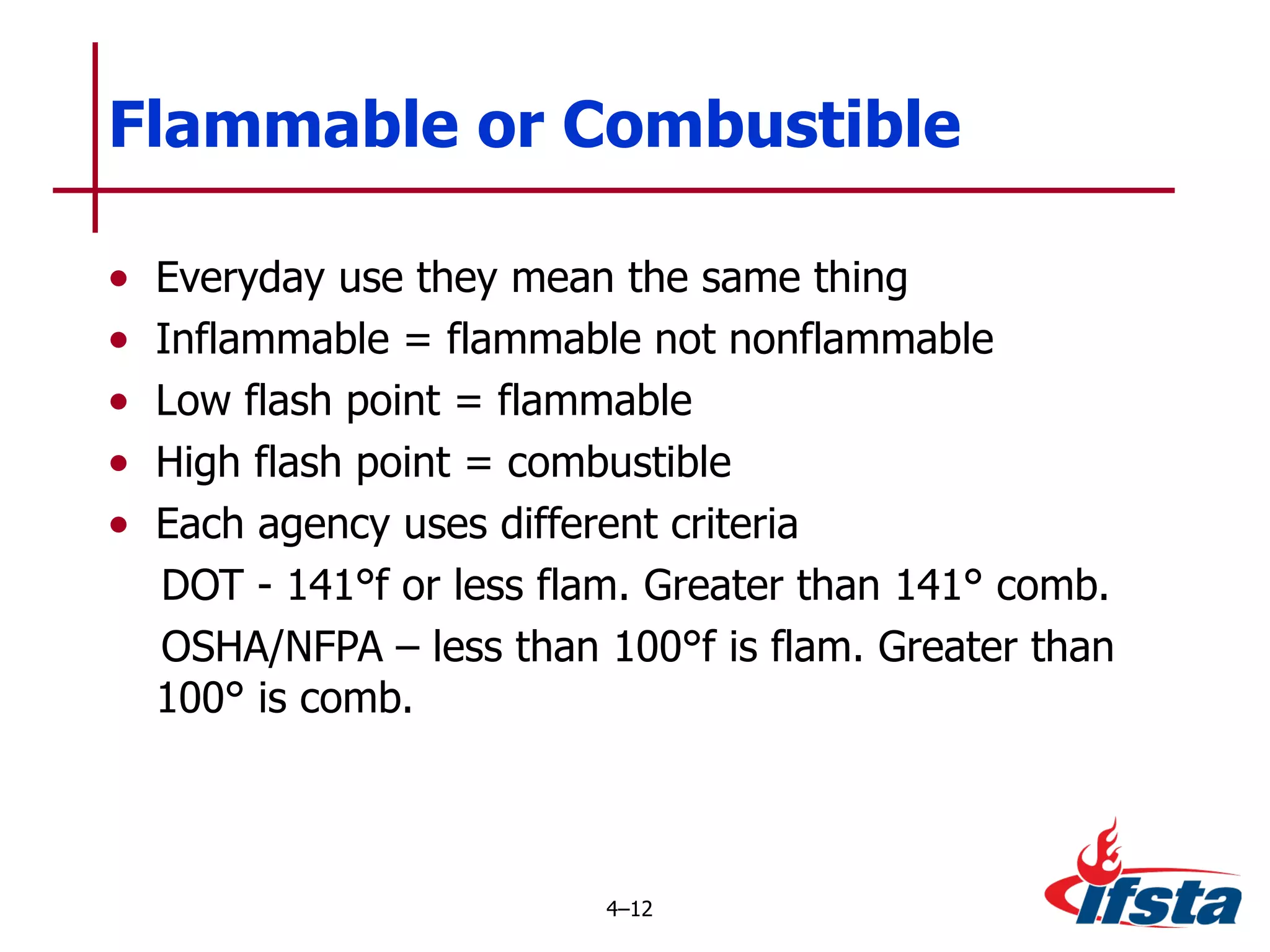
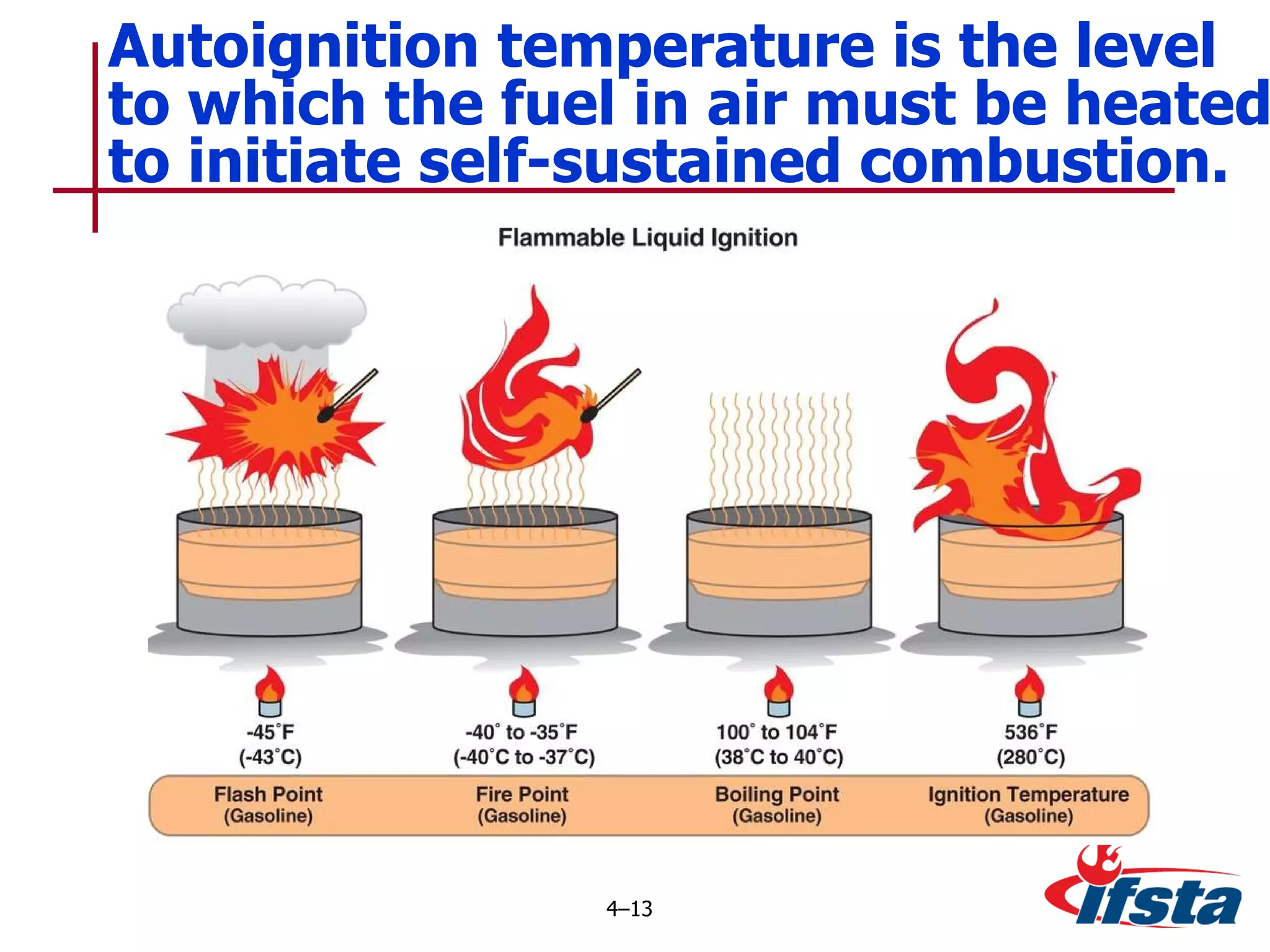
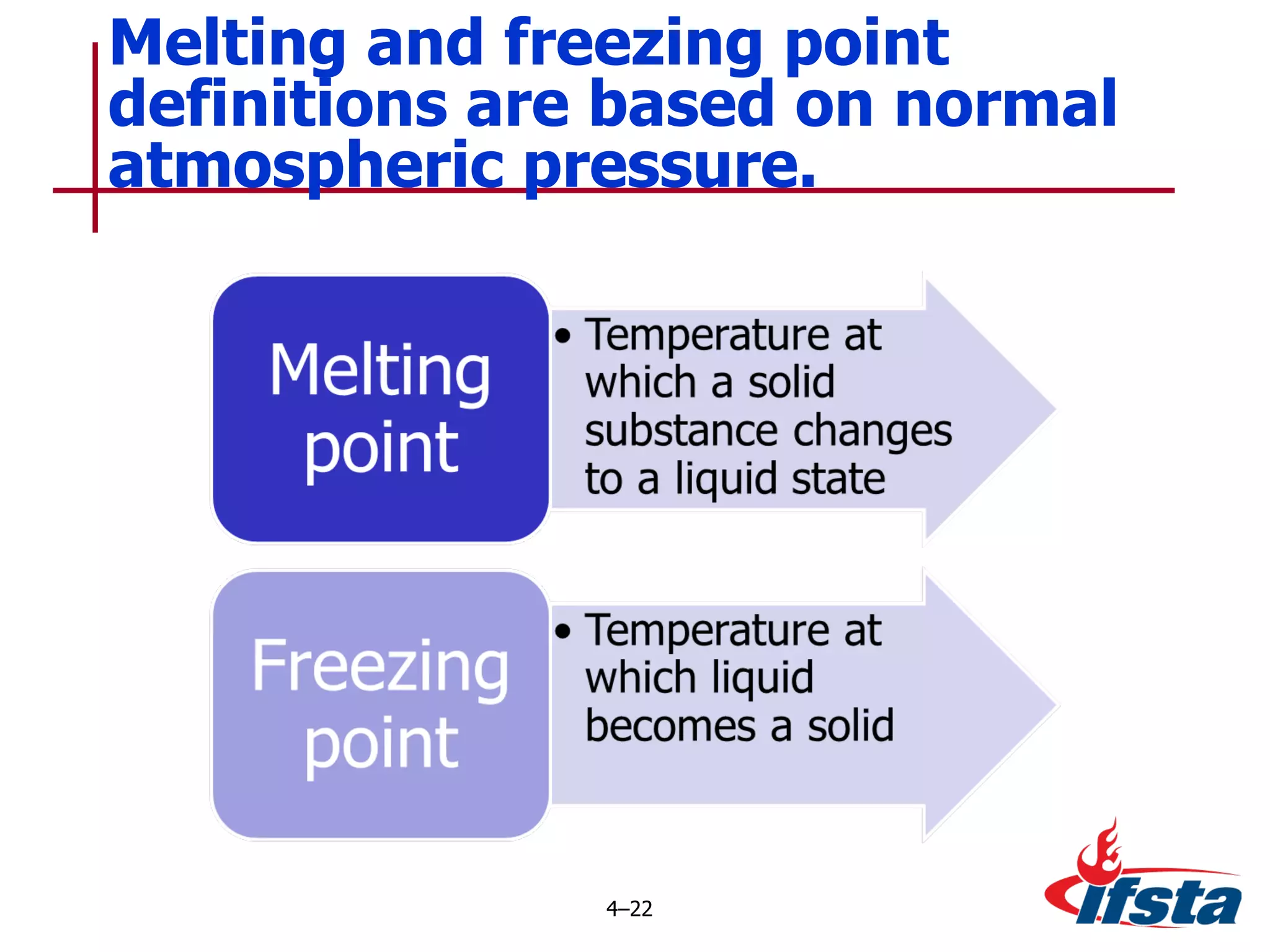
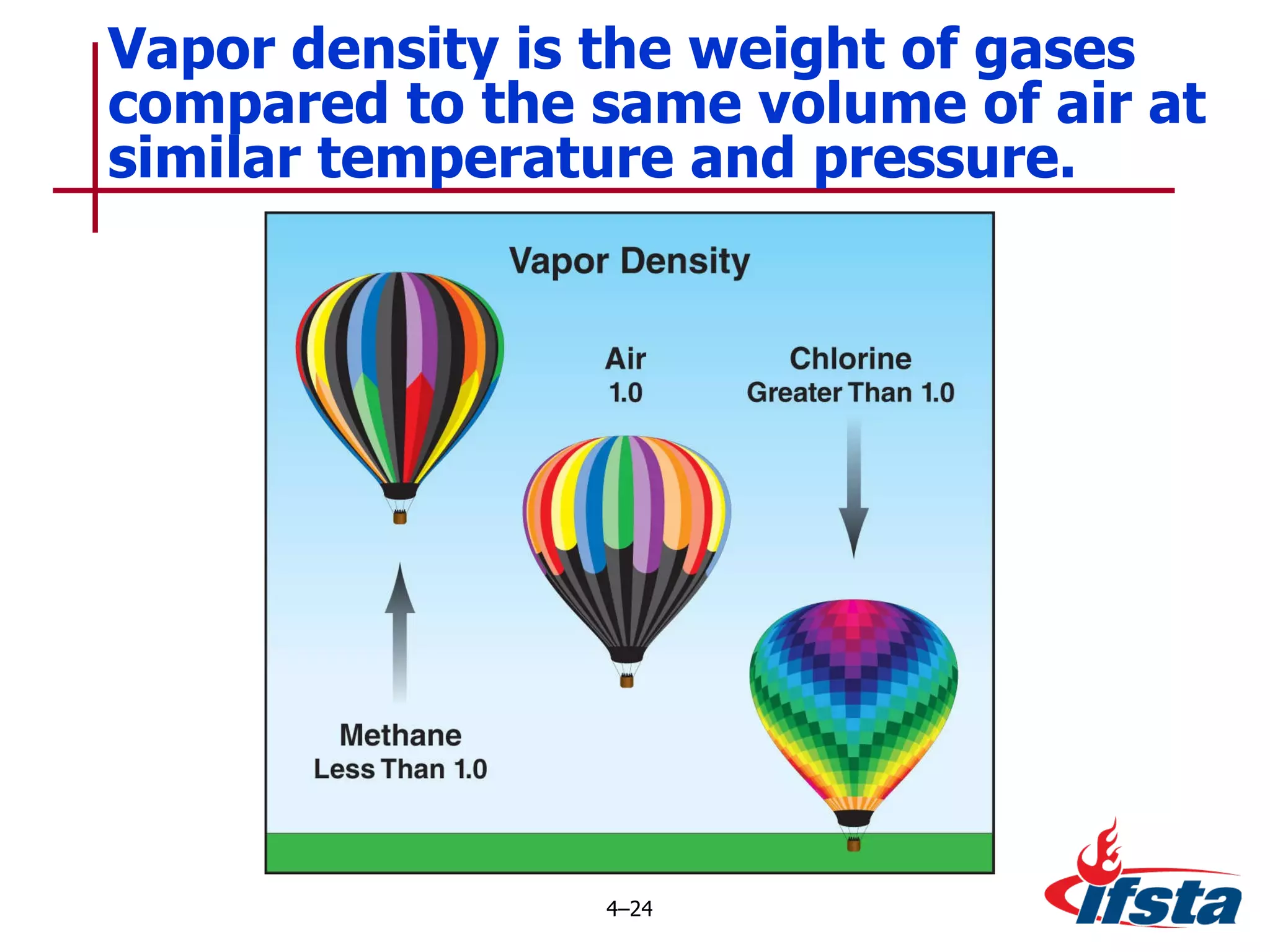
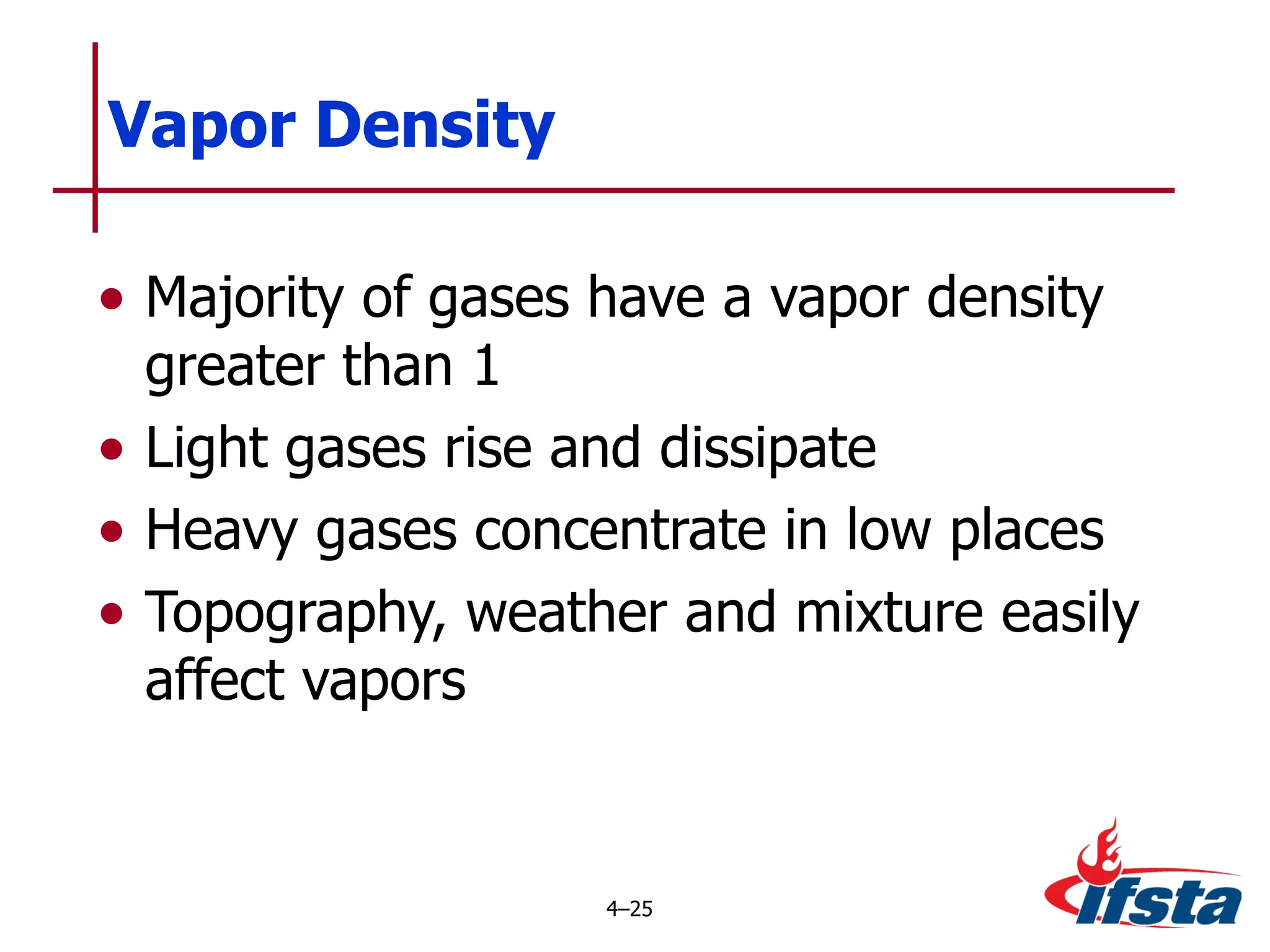
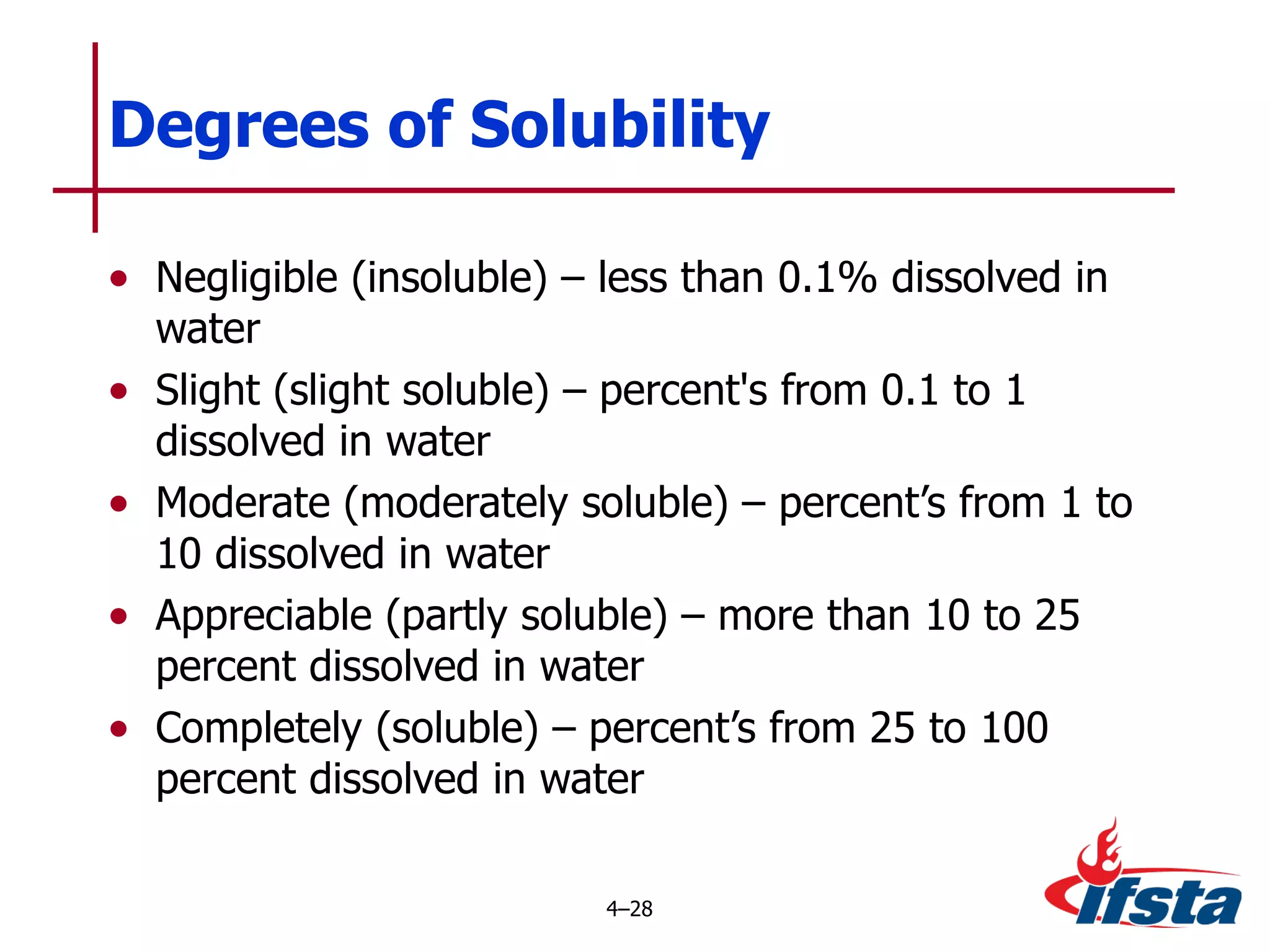
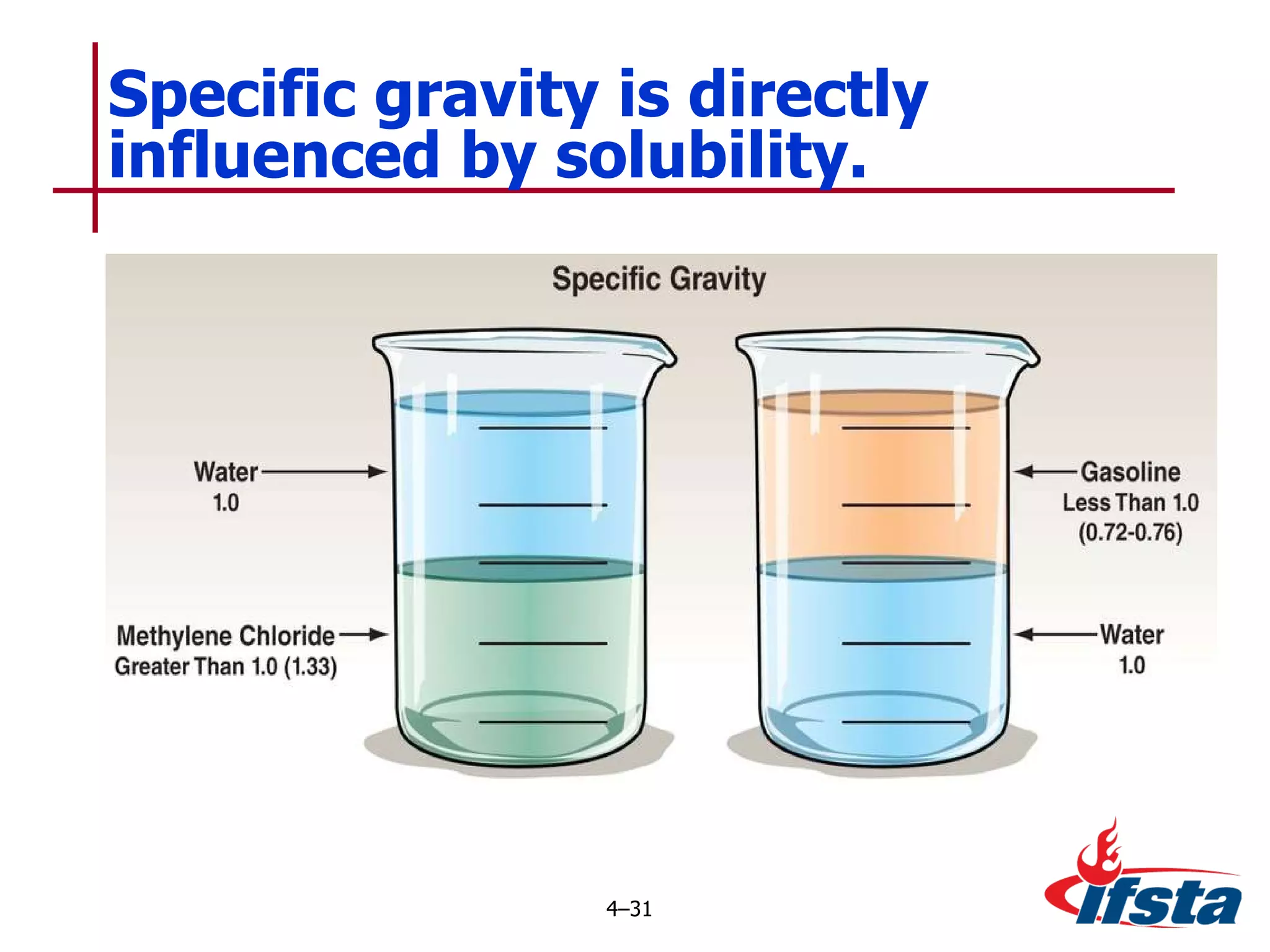
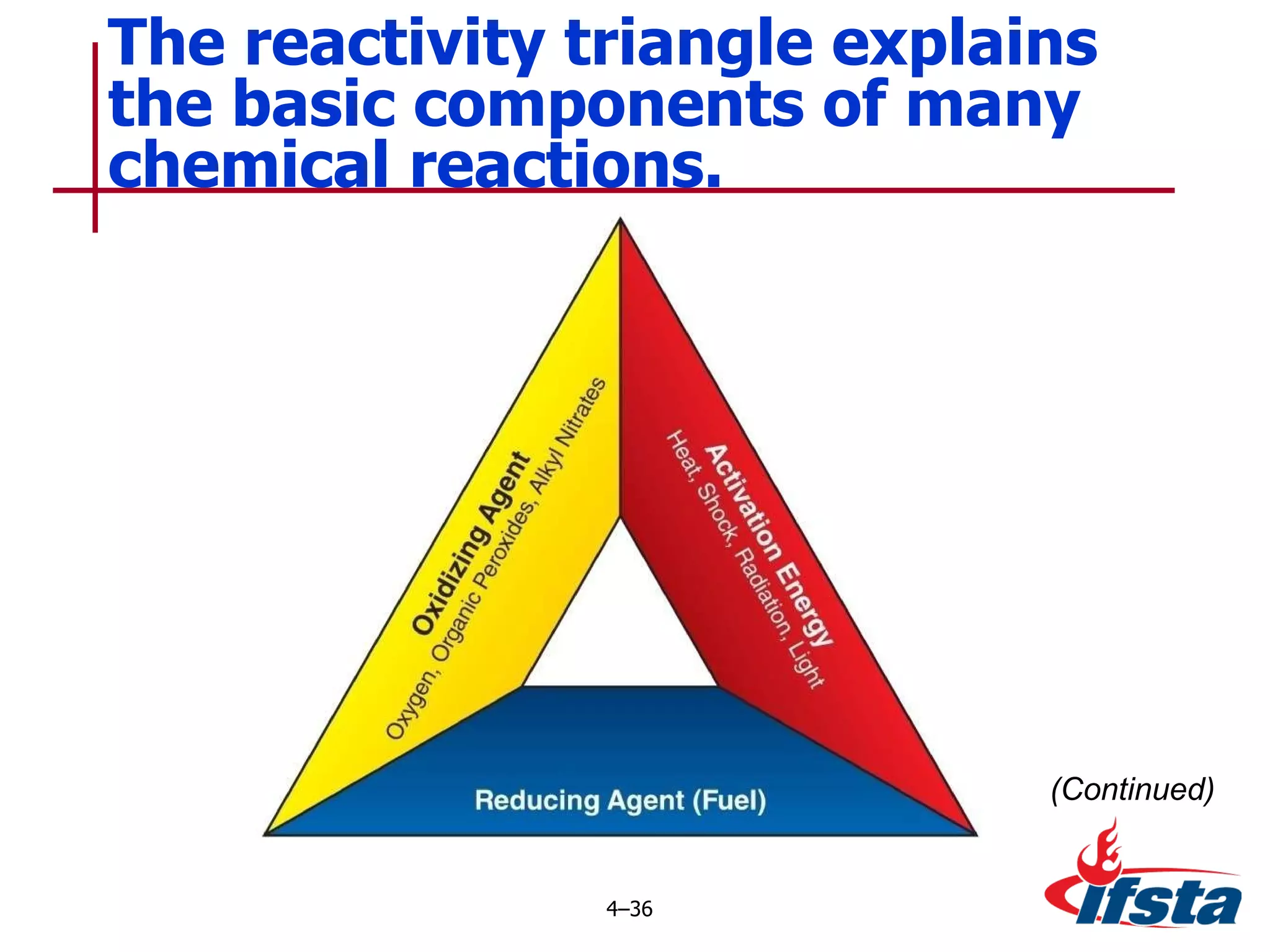
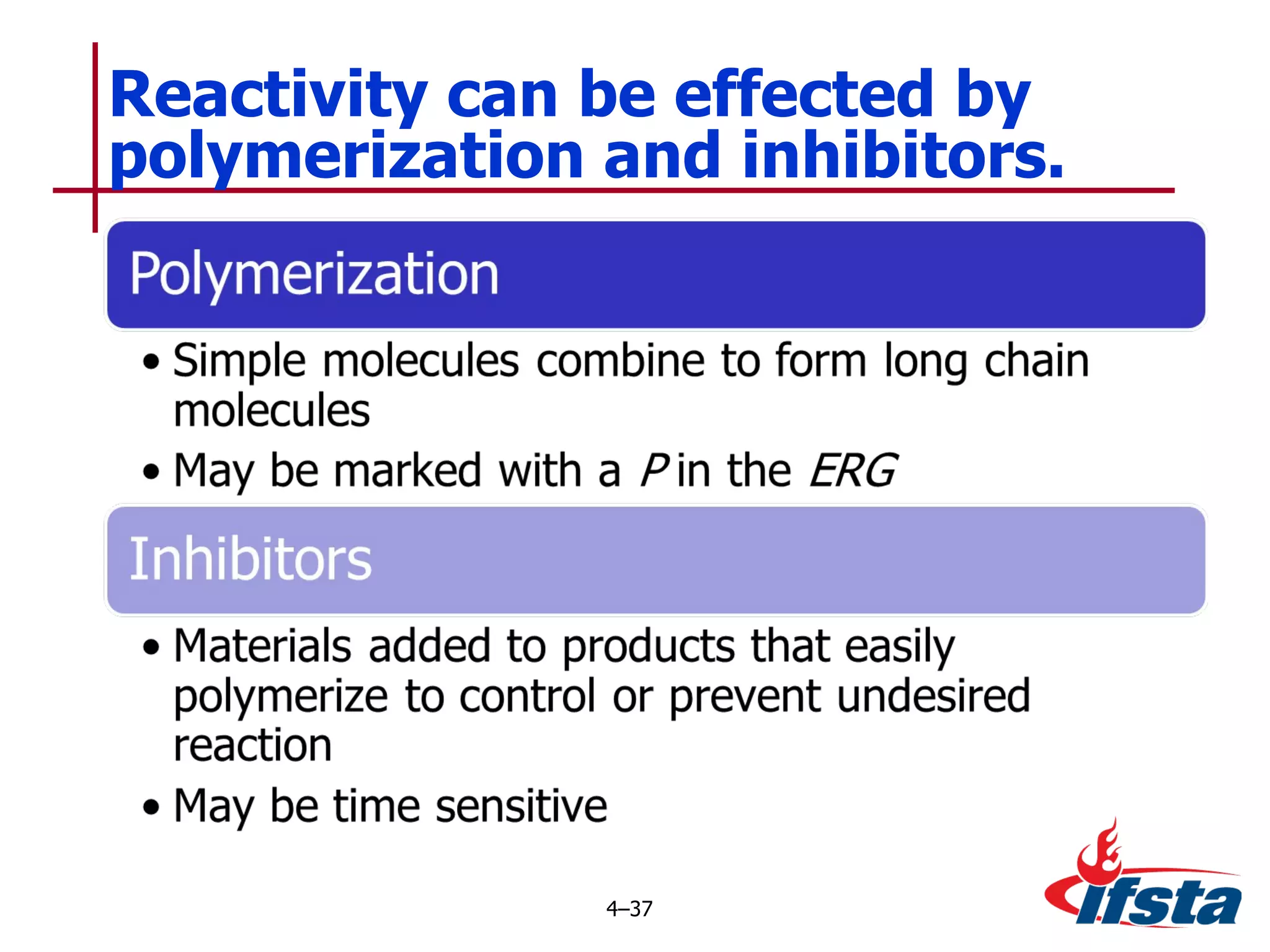
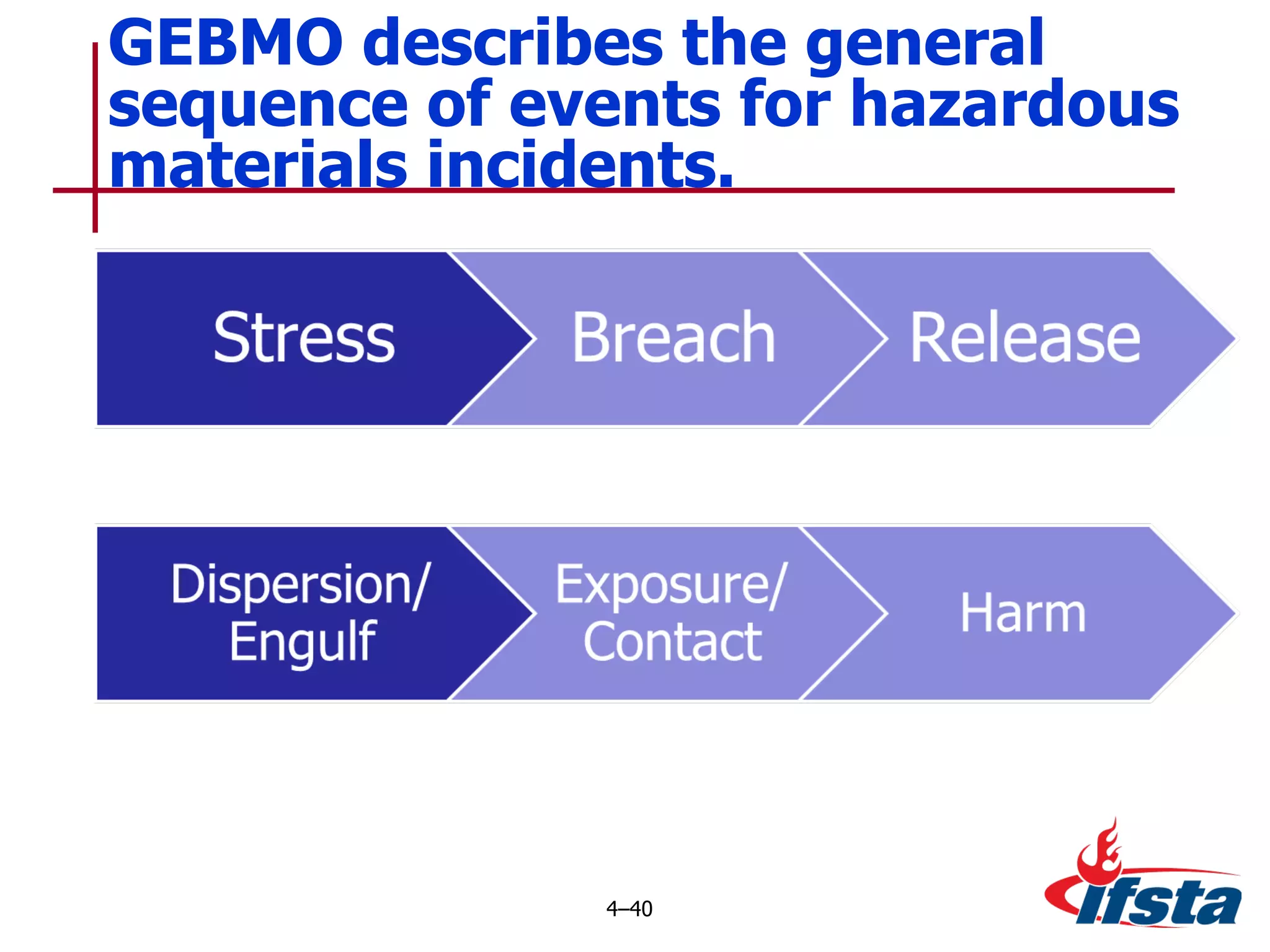
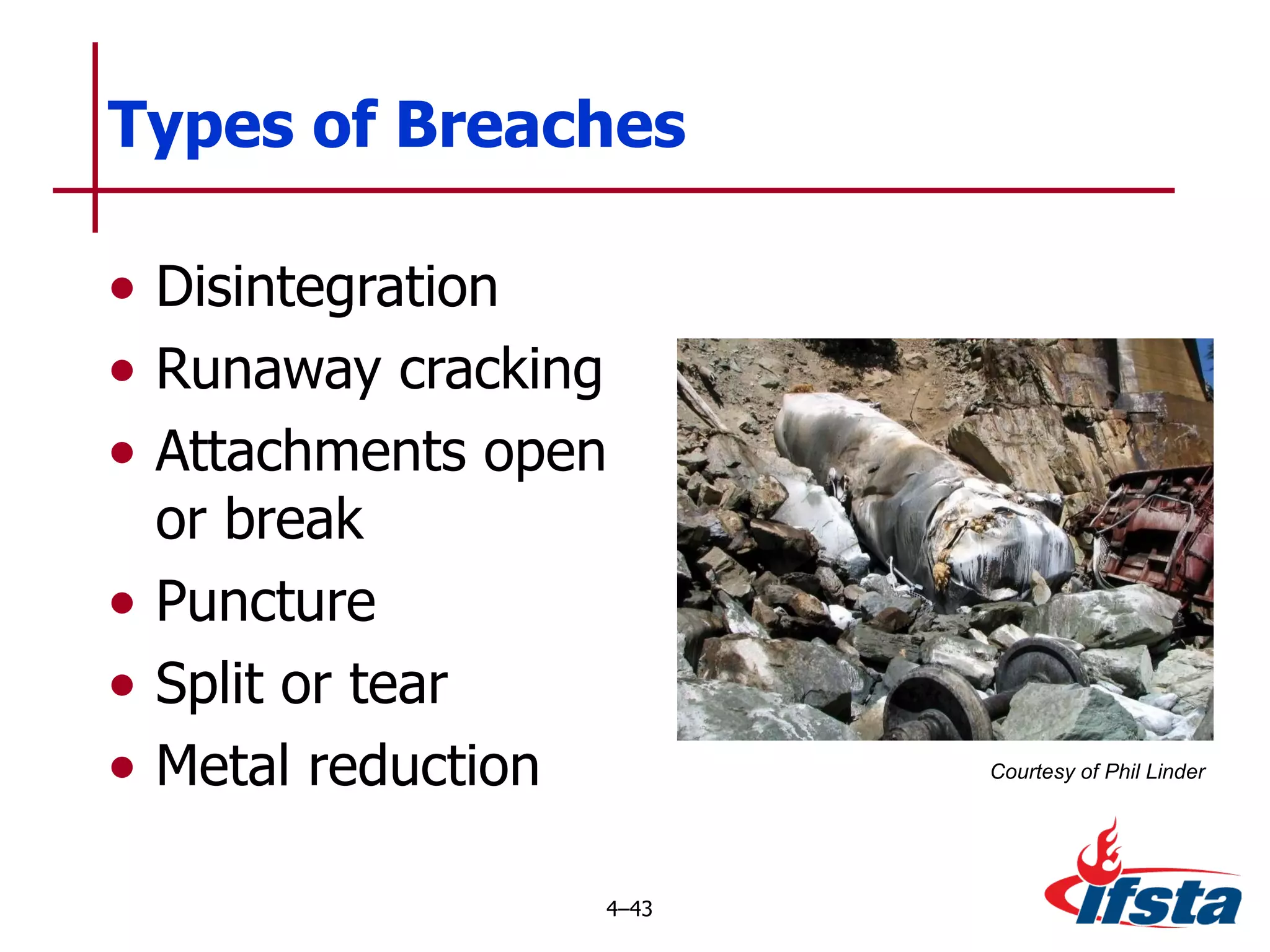
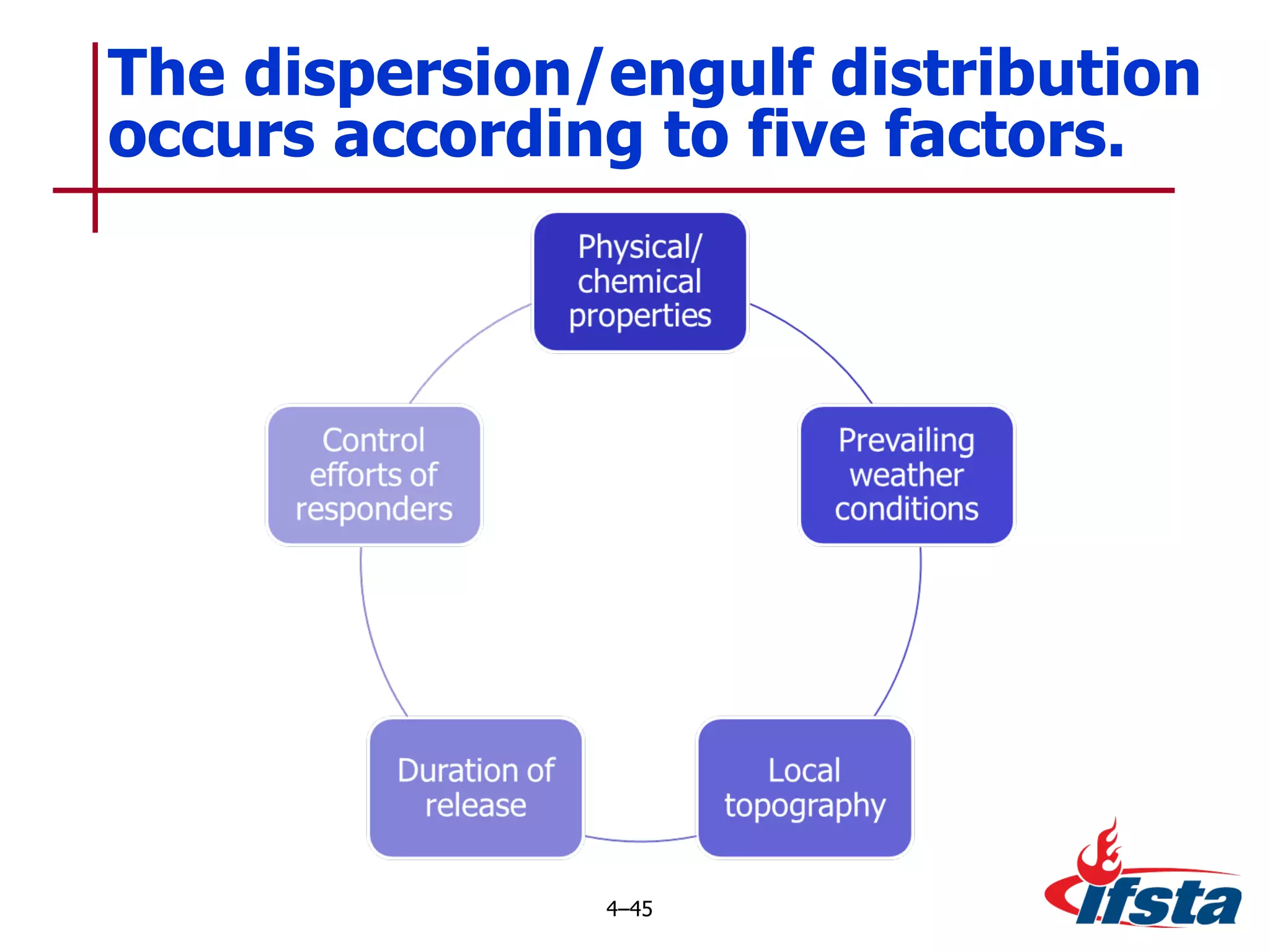
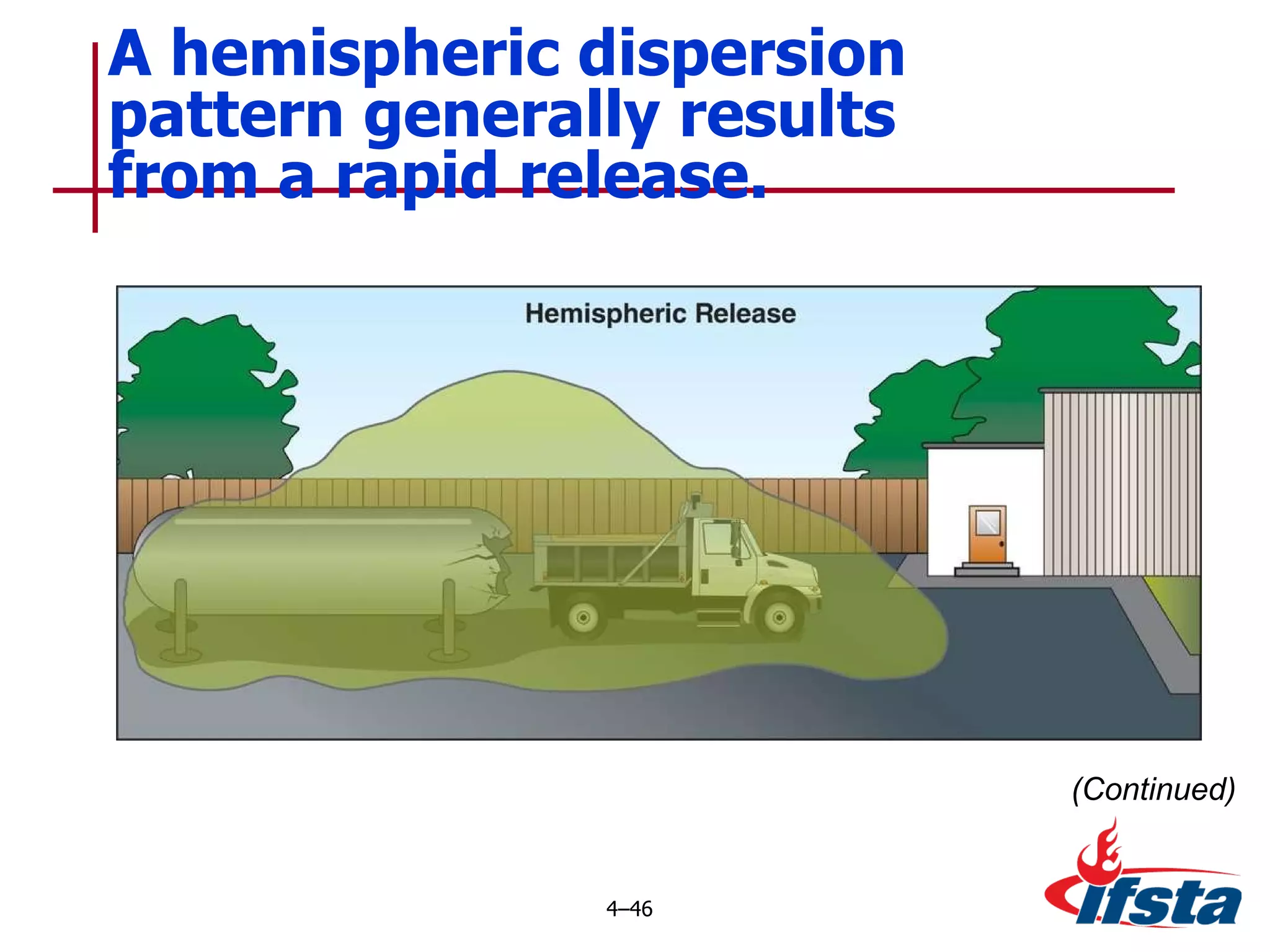
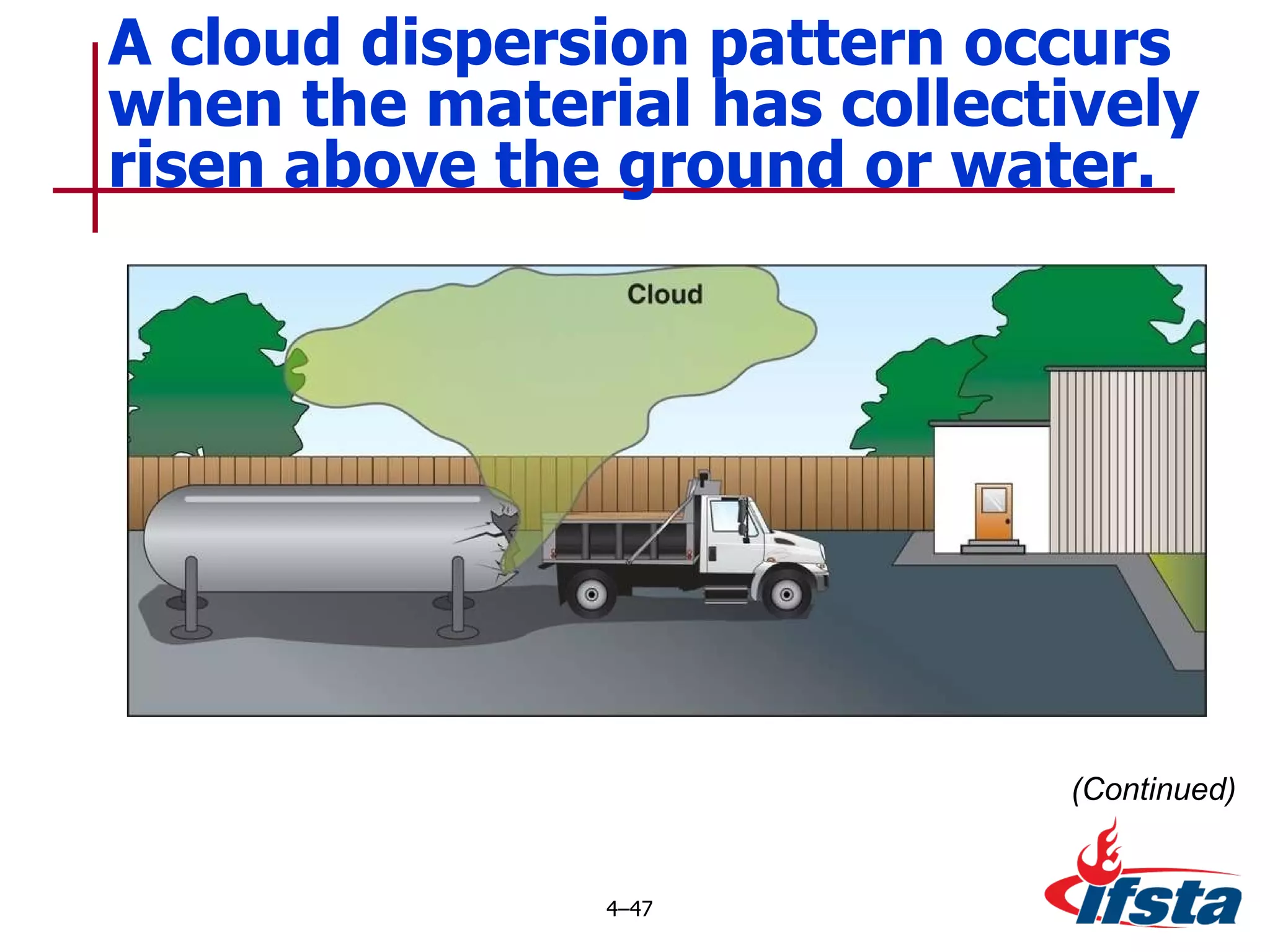
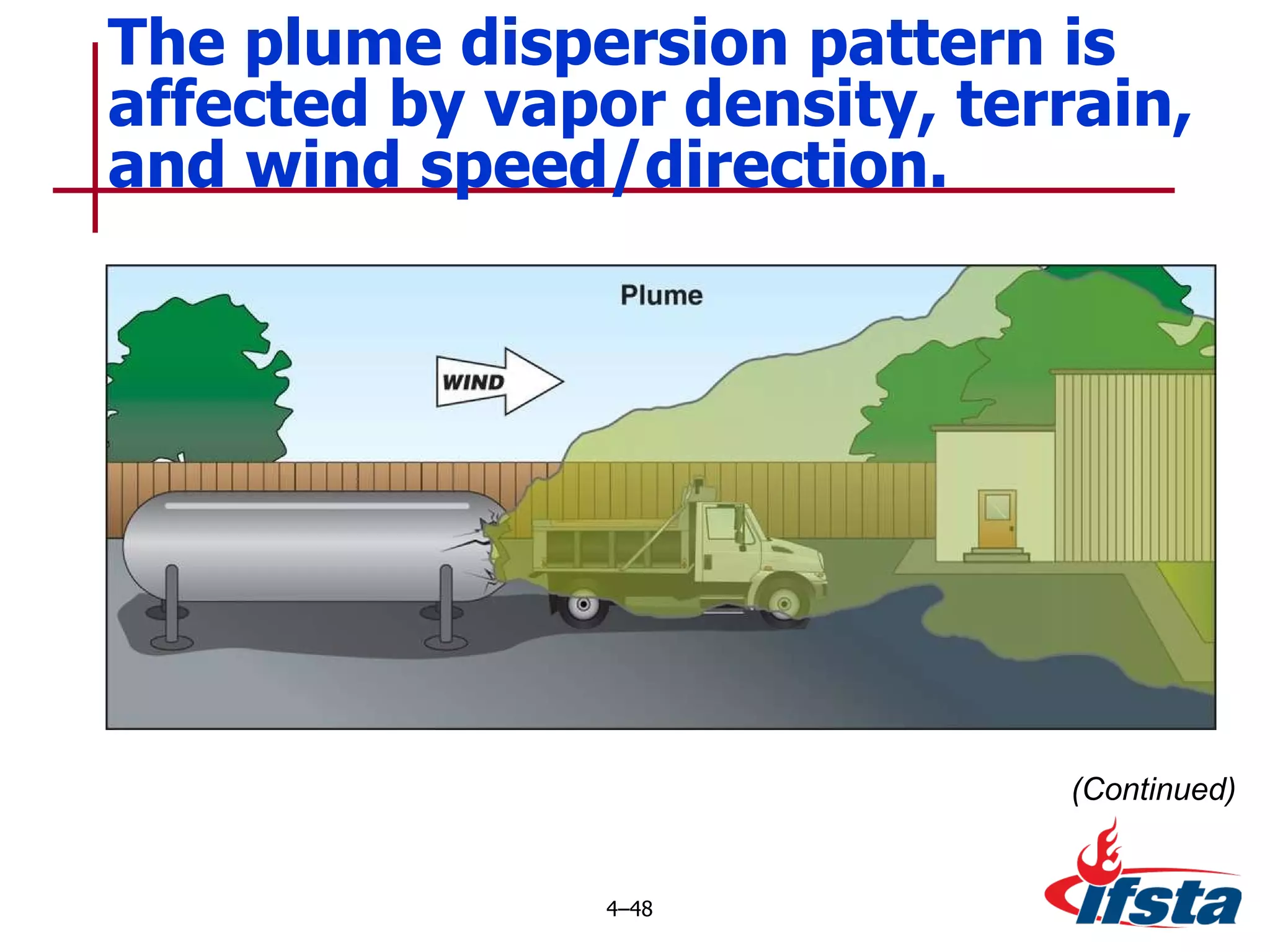
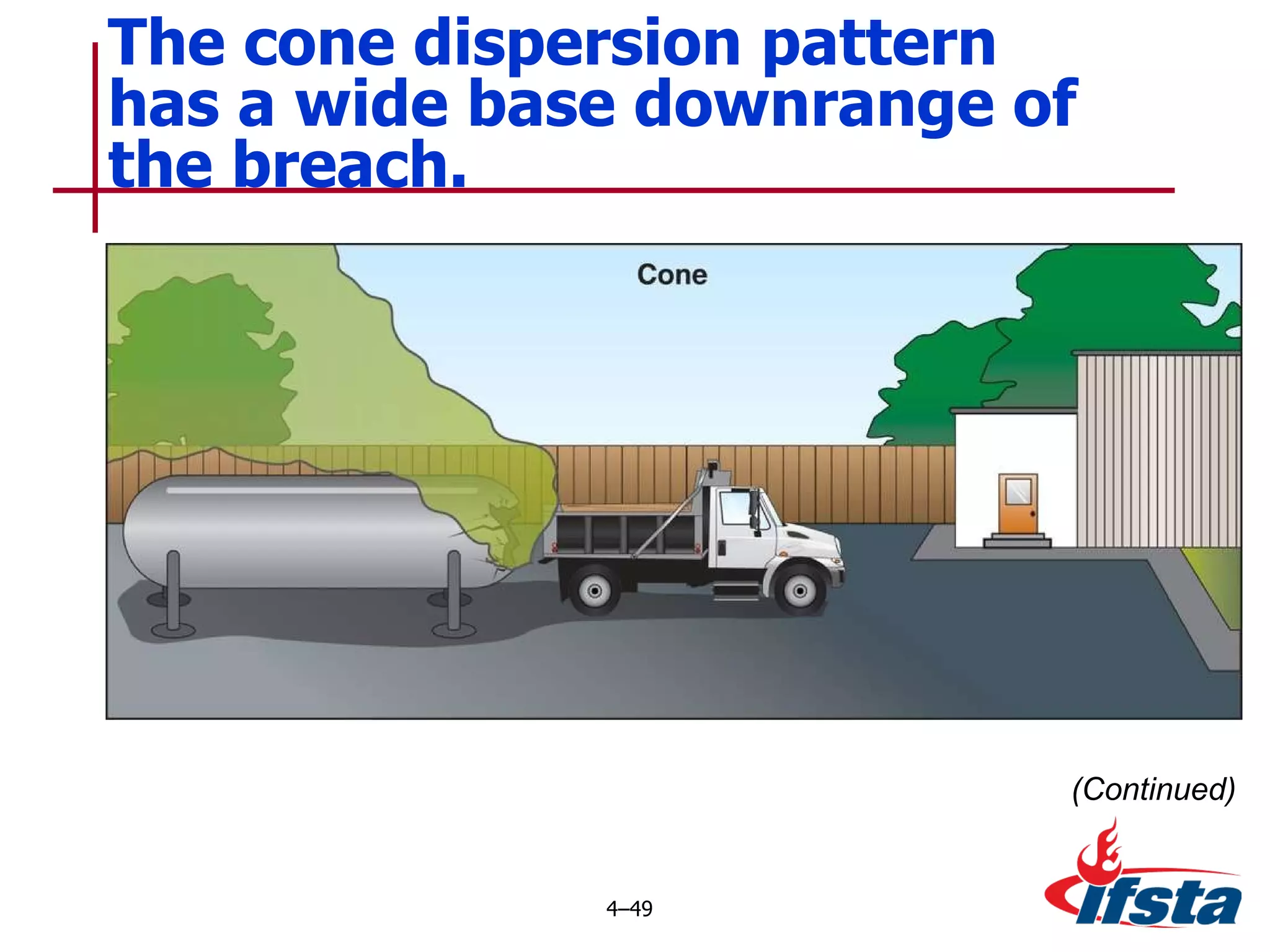
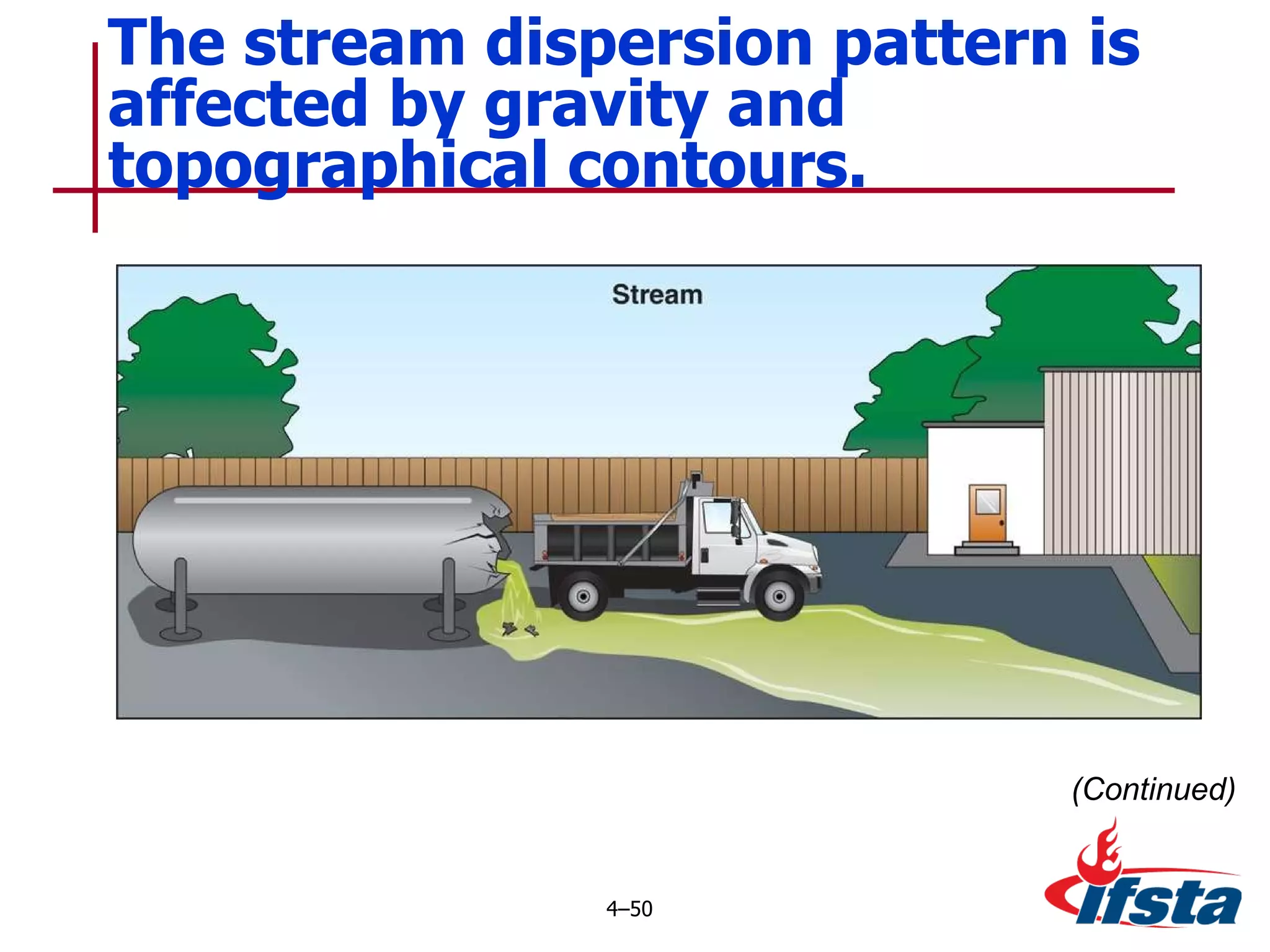
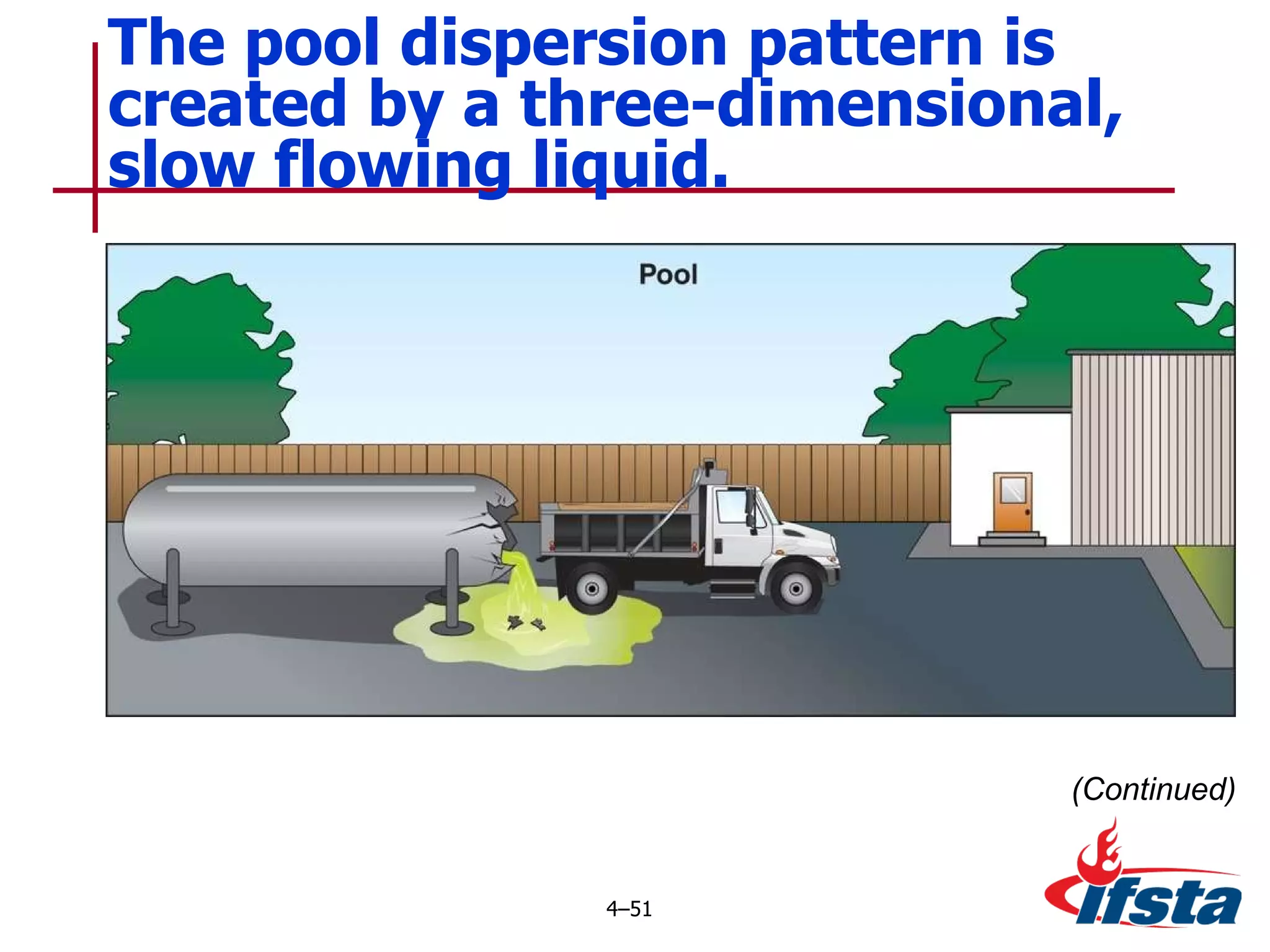
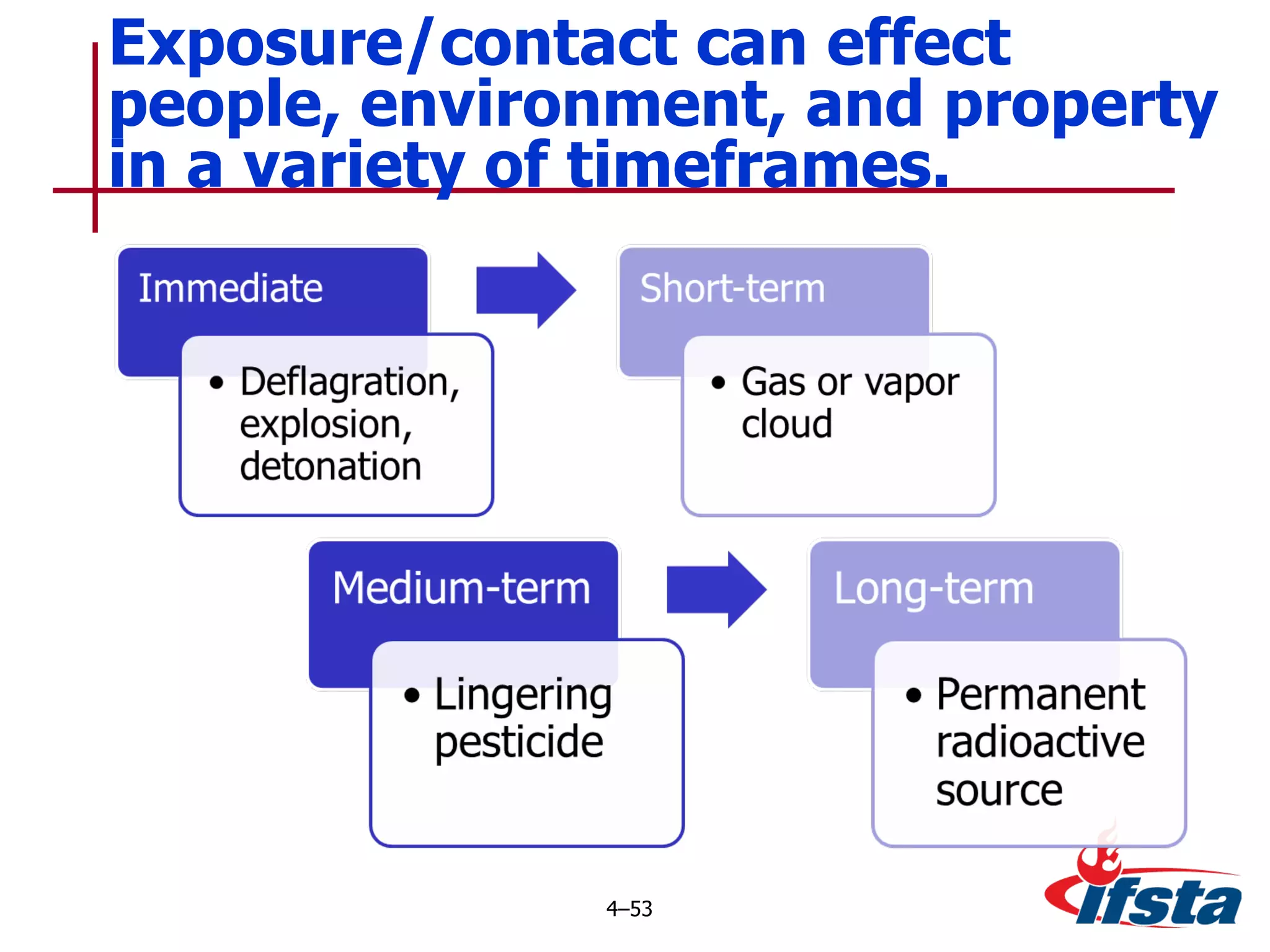
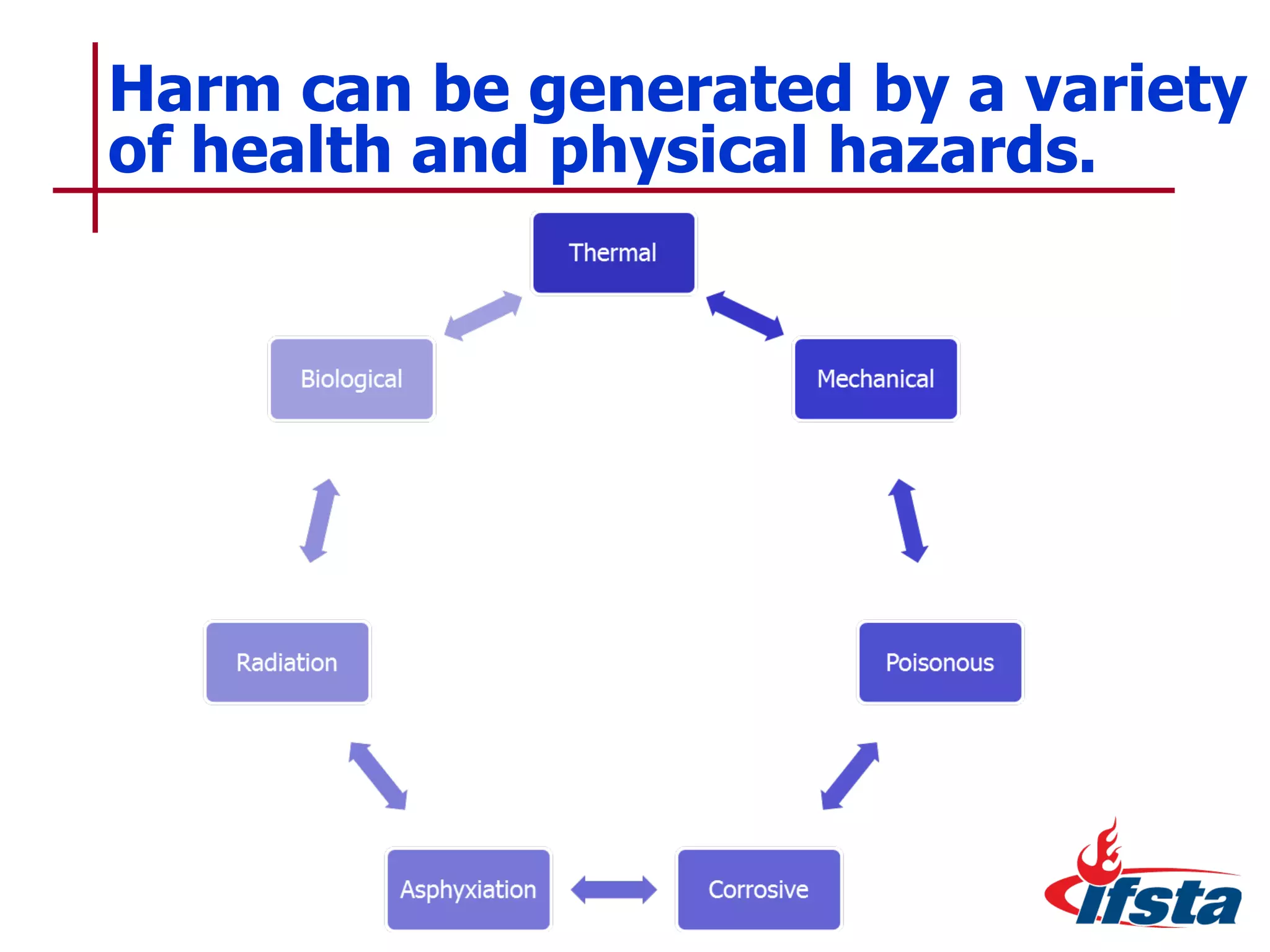
This chapter discusses the importance of understanding chemical properties and how they determine hazardous material behavior for first responders. Key physical properties covered include states of matter, particle types, flammability limits, vapor pressure, boiling point, solubility, reactivity, and more. Understanding these properties helps predict how a material will disperse if released and potentially harm people, property or the environment. The General Hazardous Materials Behavior Model outlines the common sequence of events from a container breach to exposure.