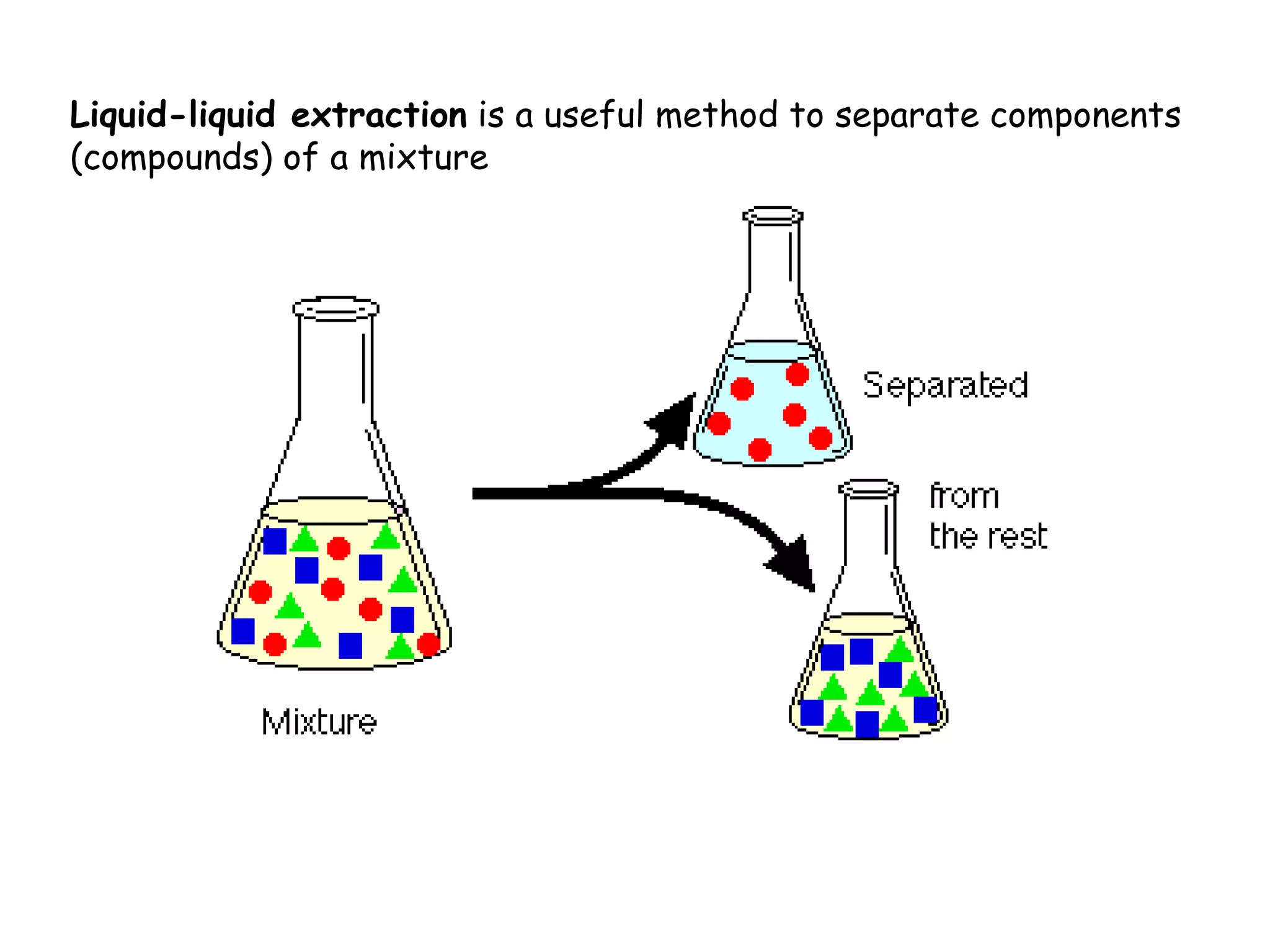
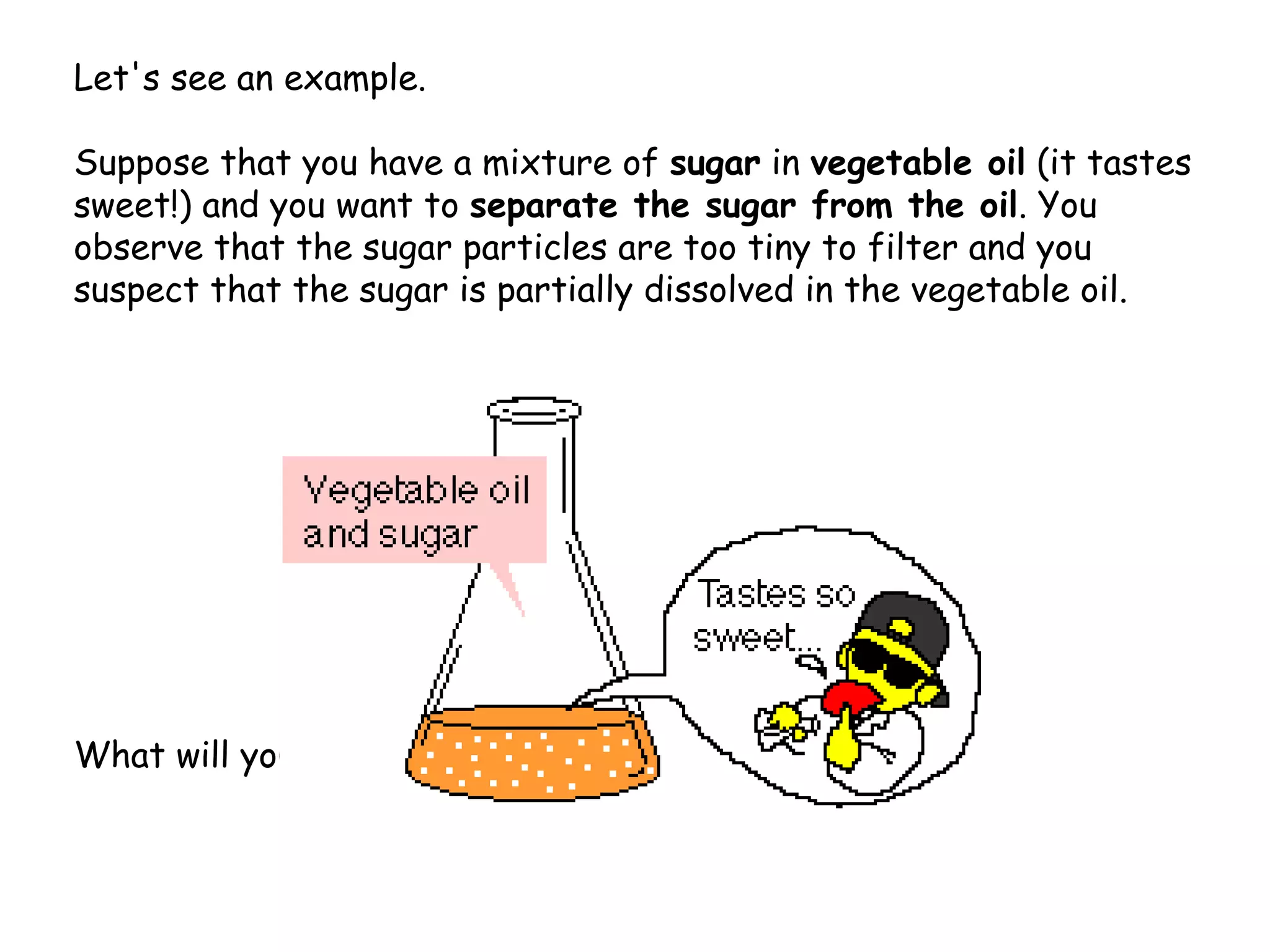
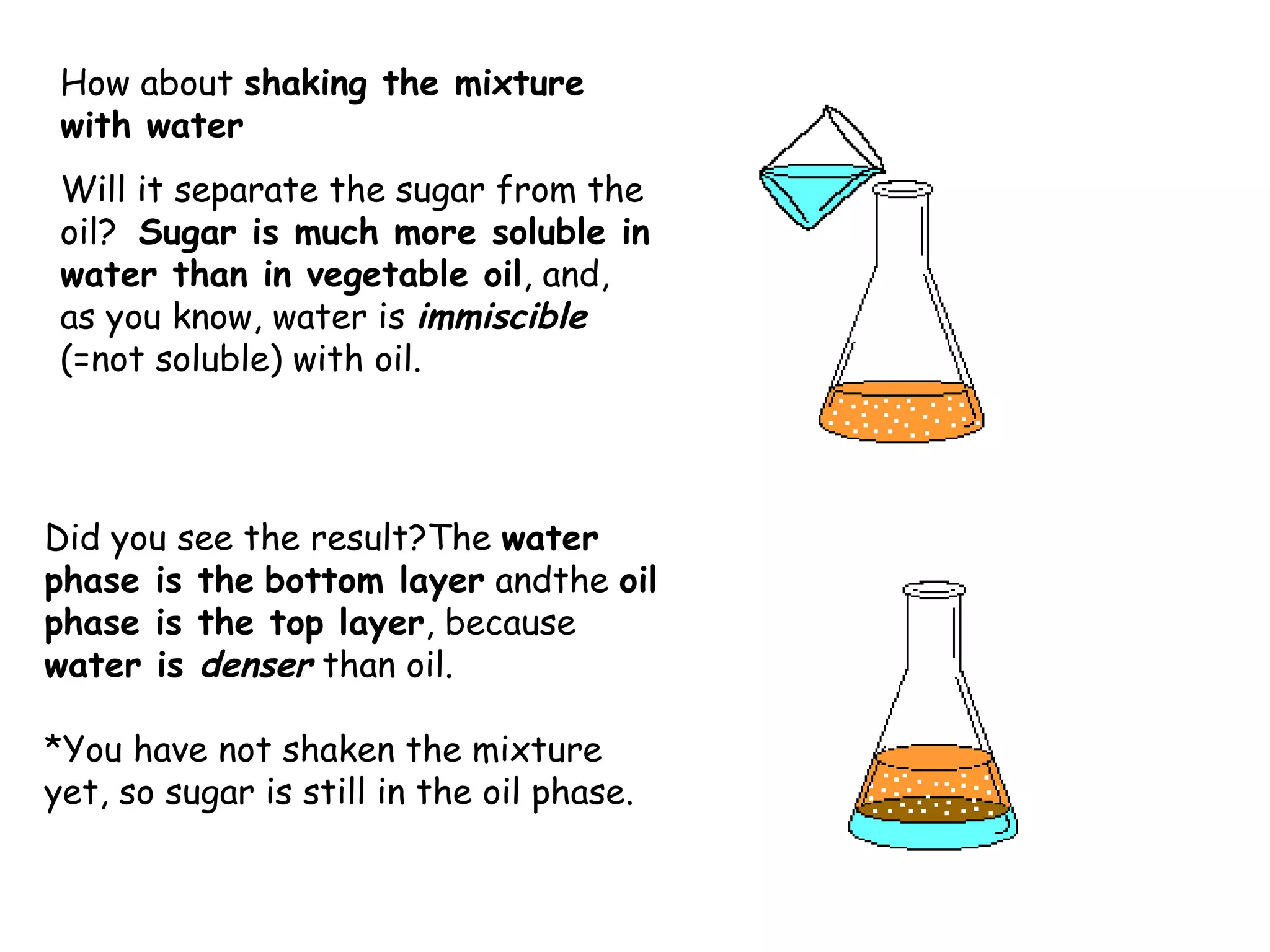
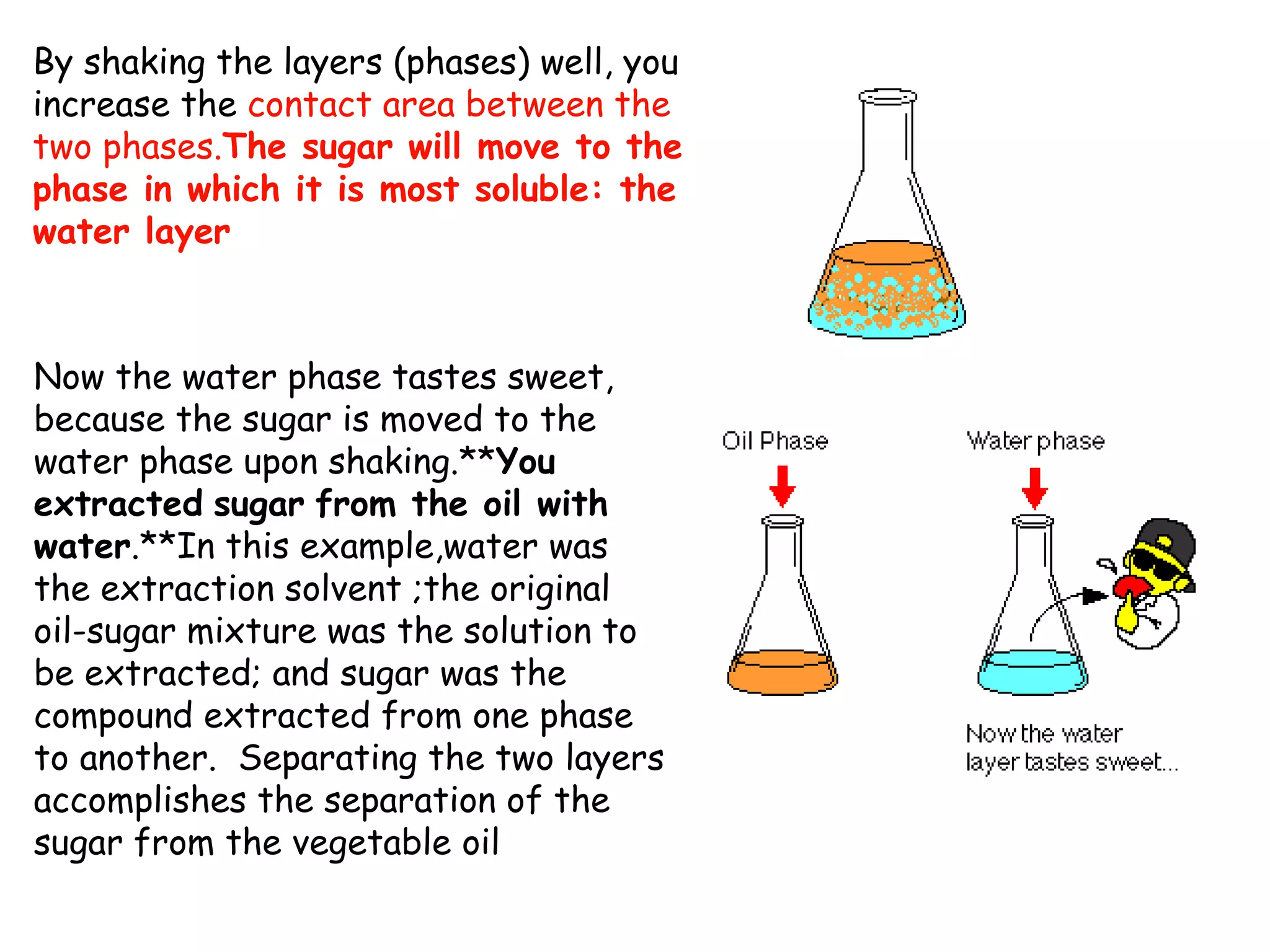
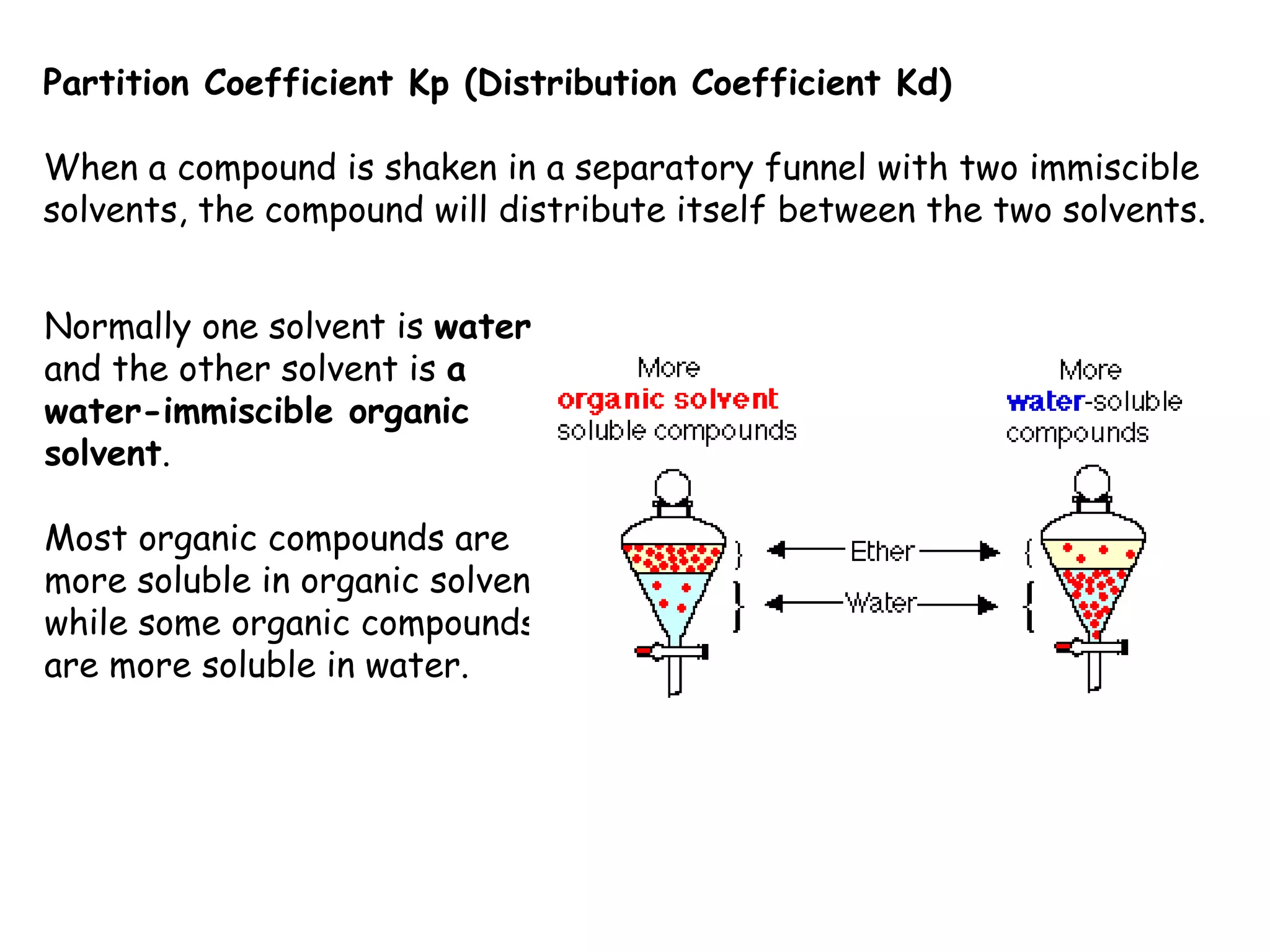
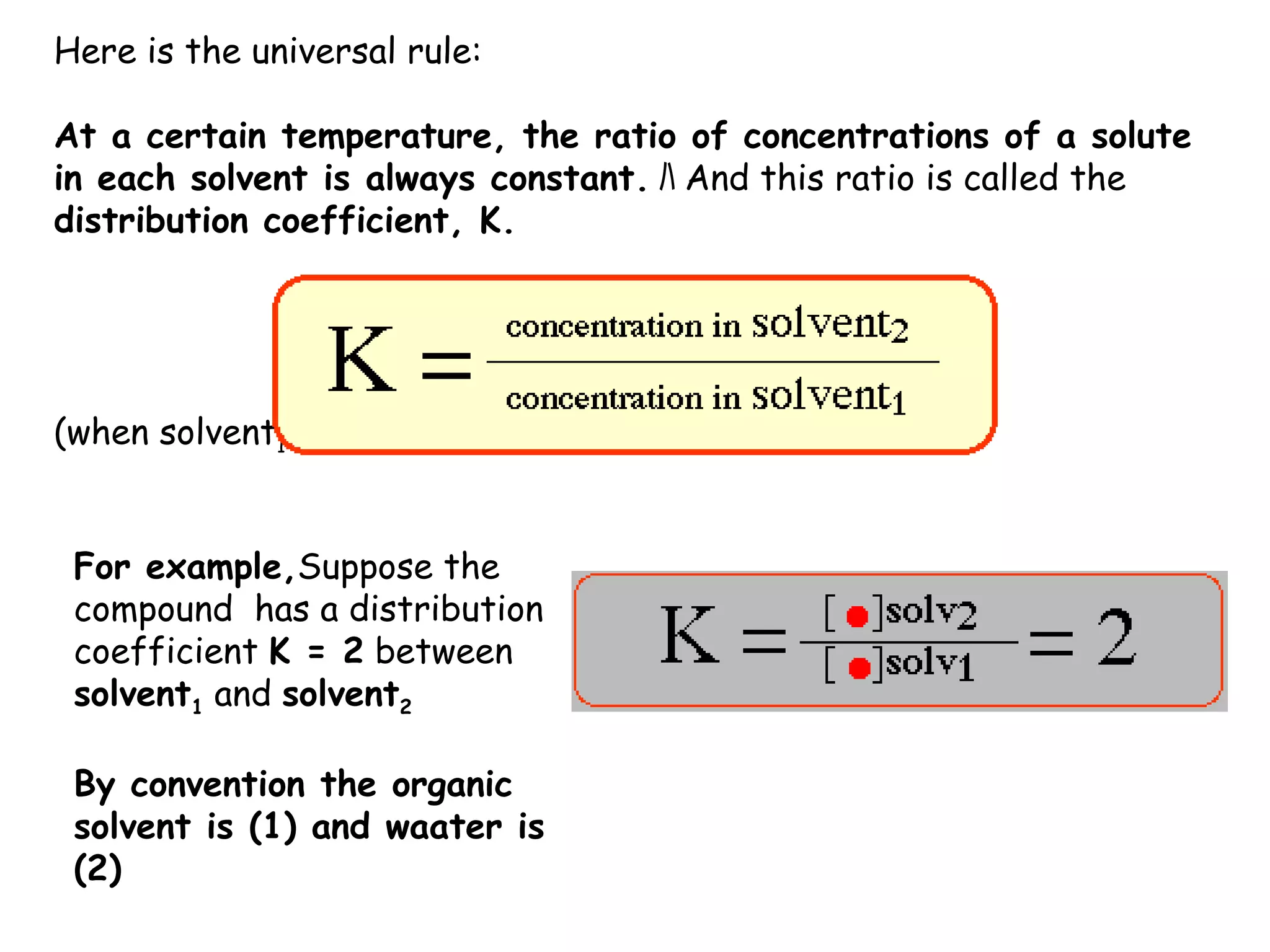
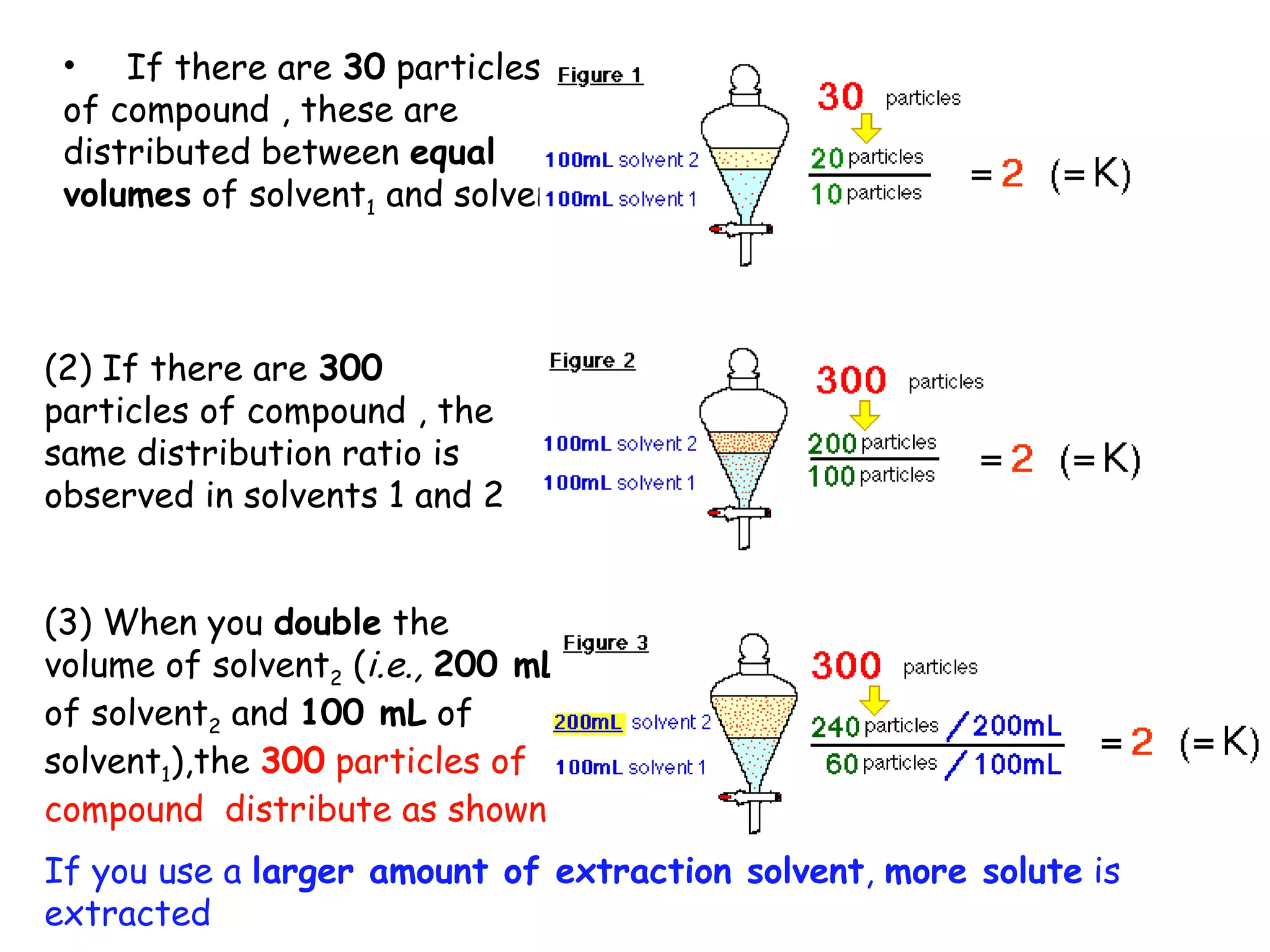
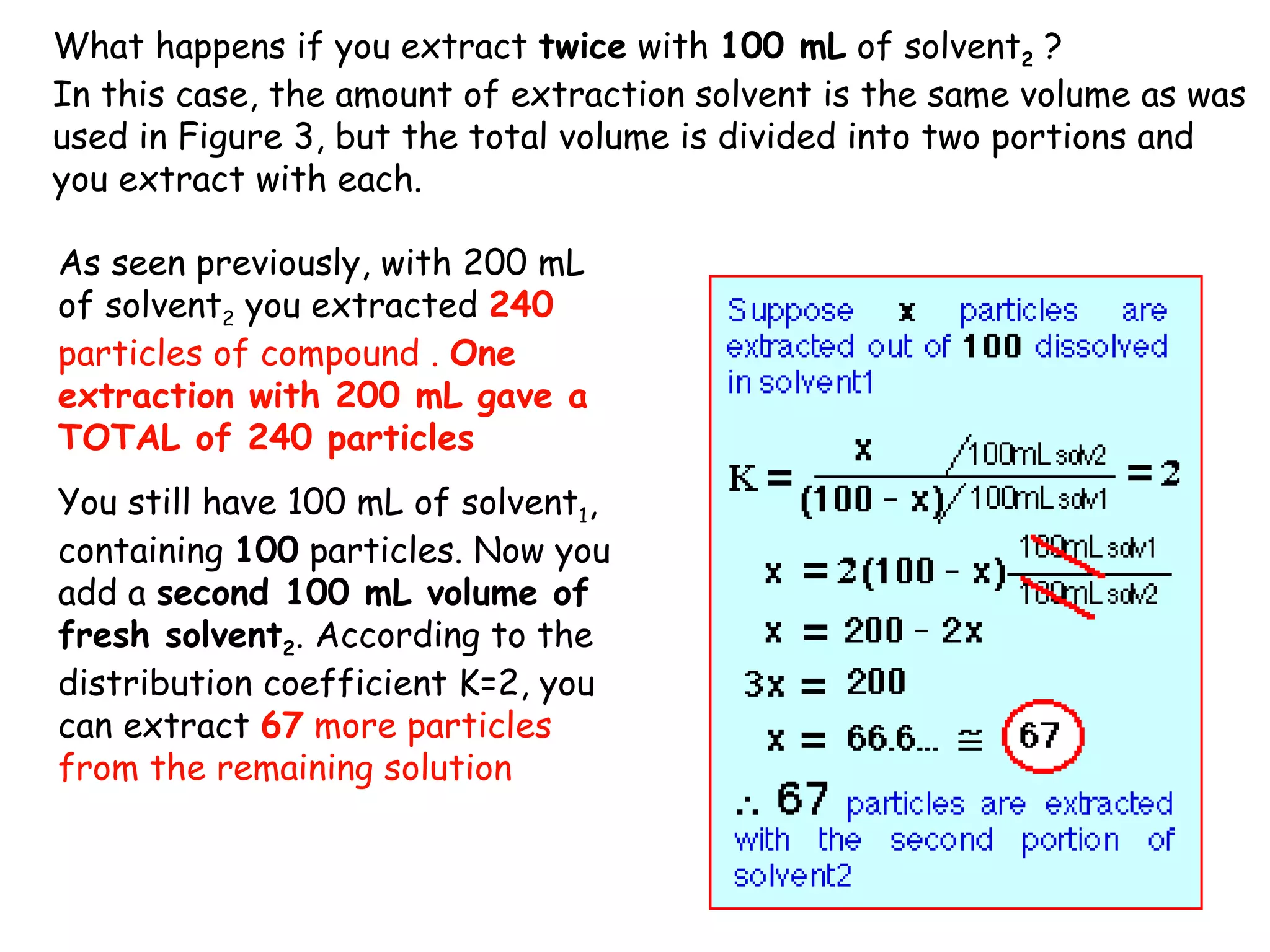
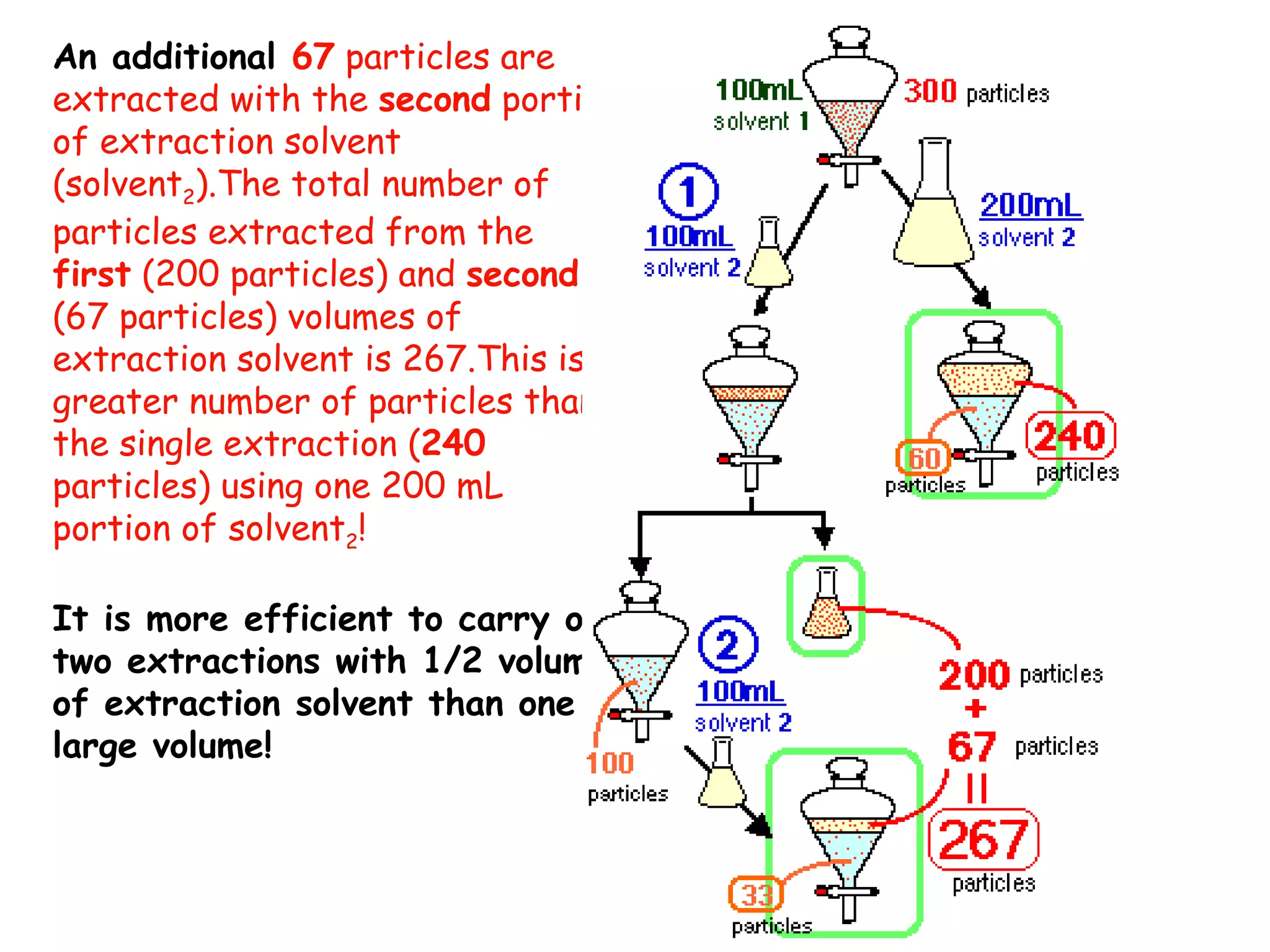
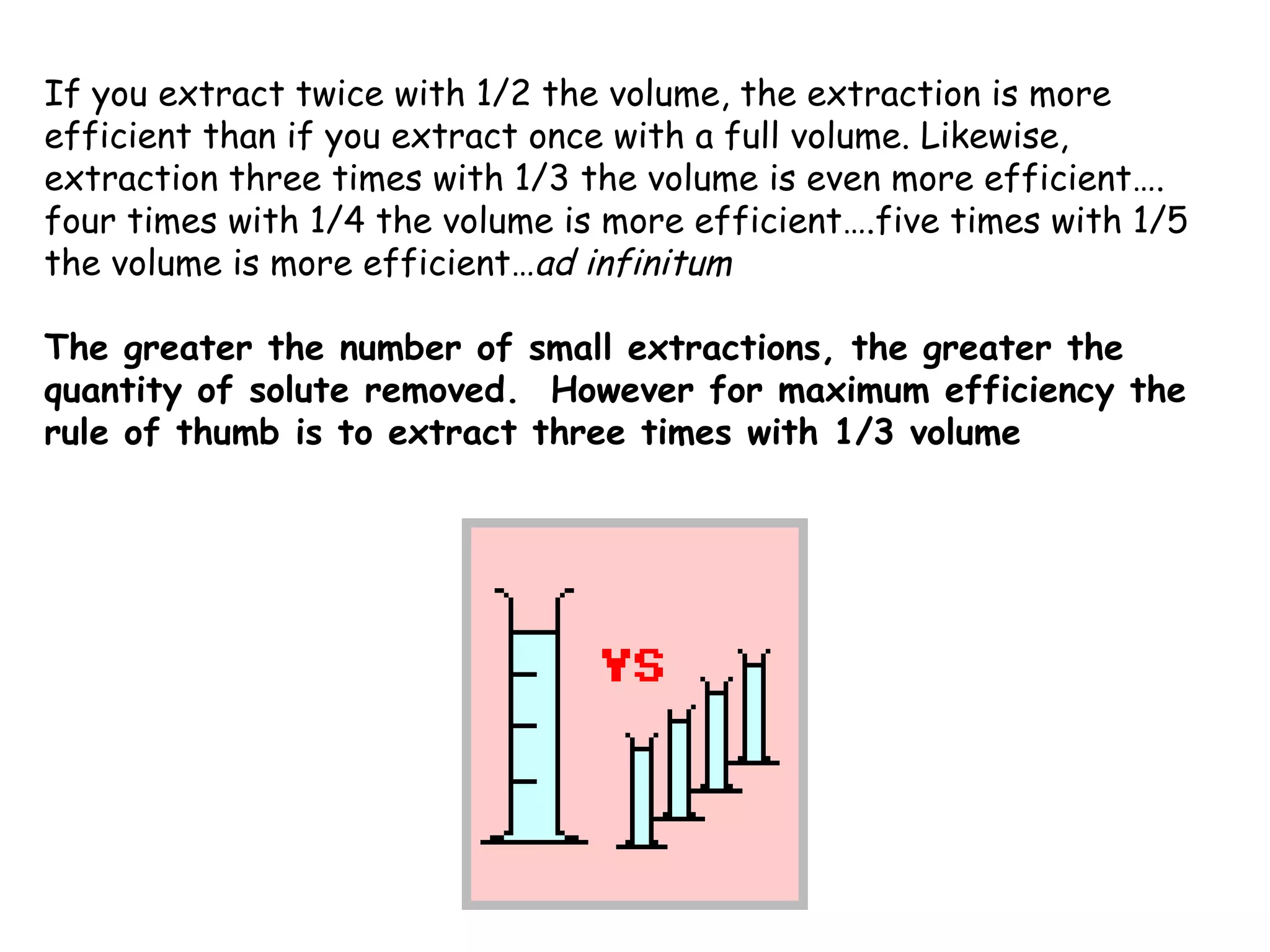
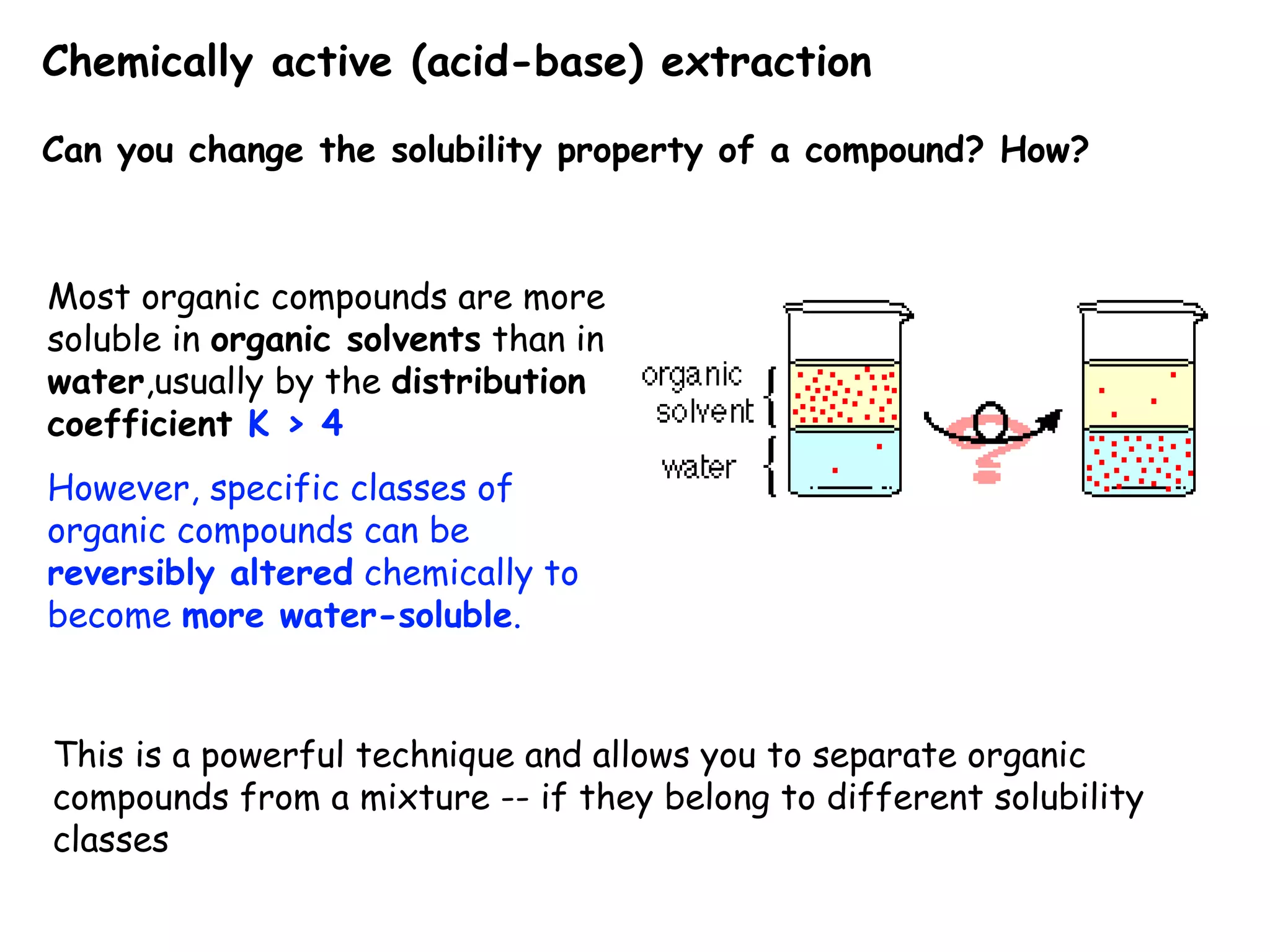
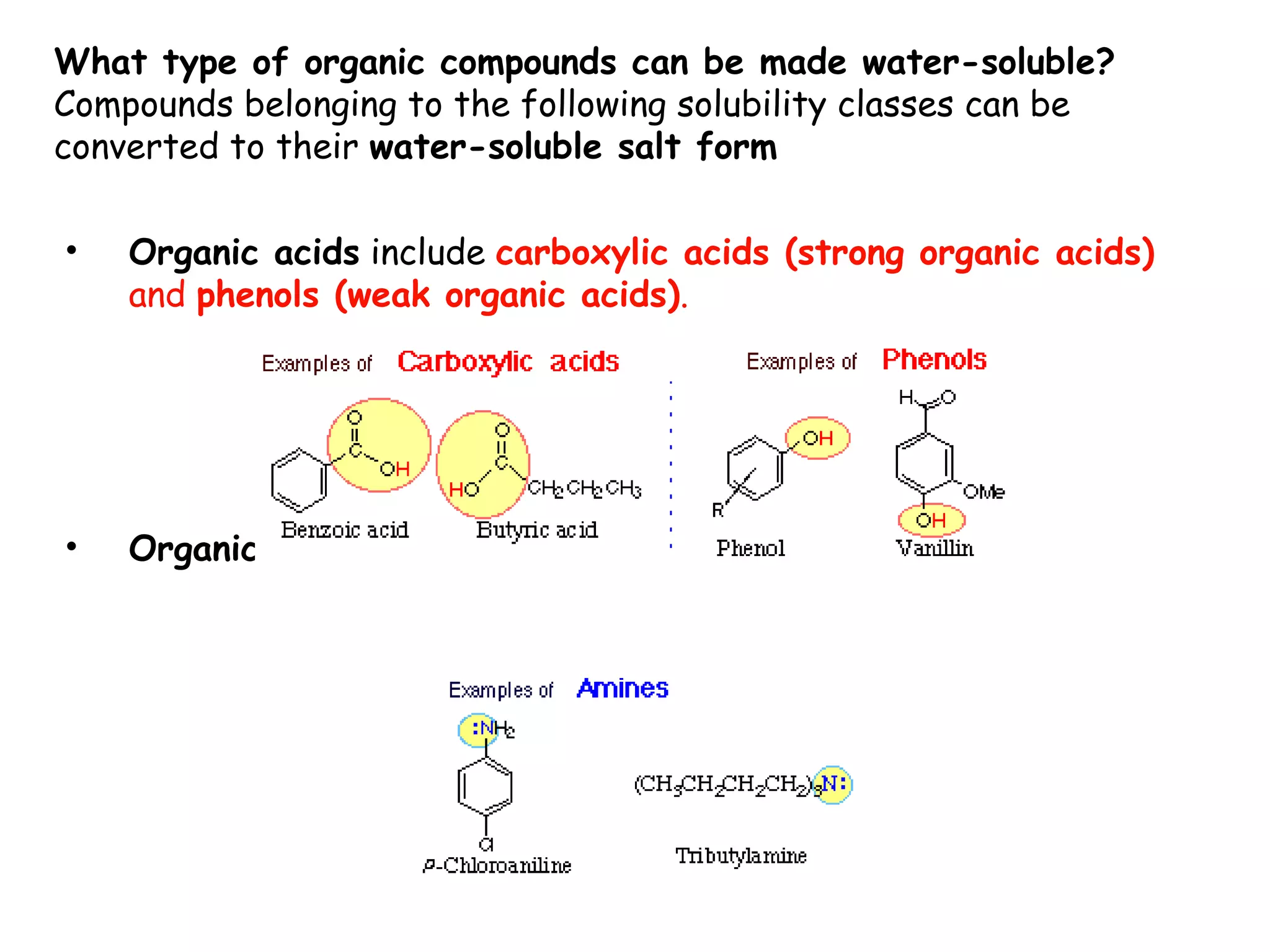
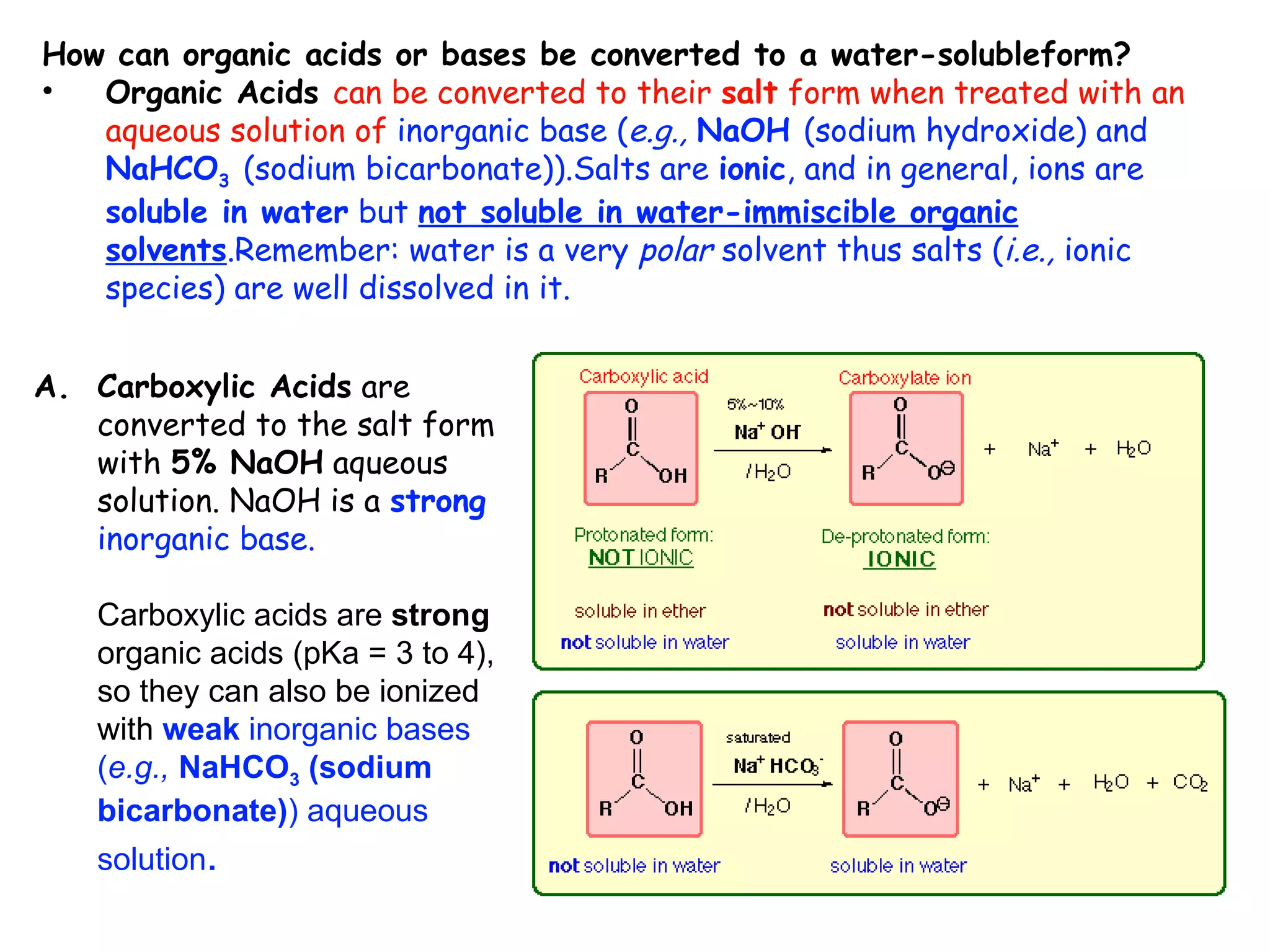
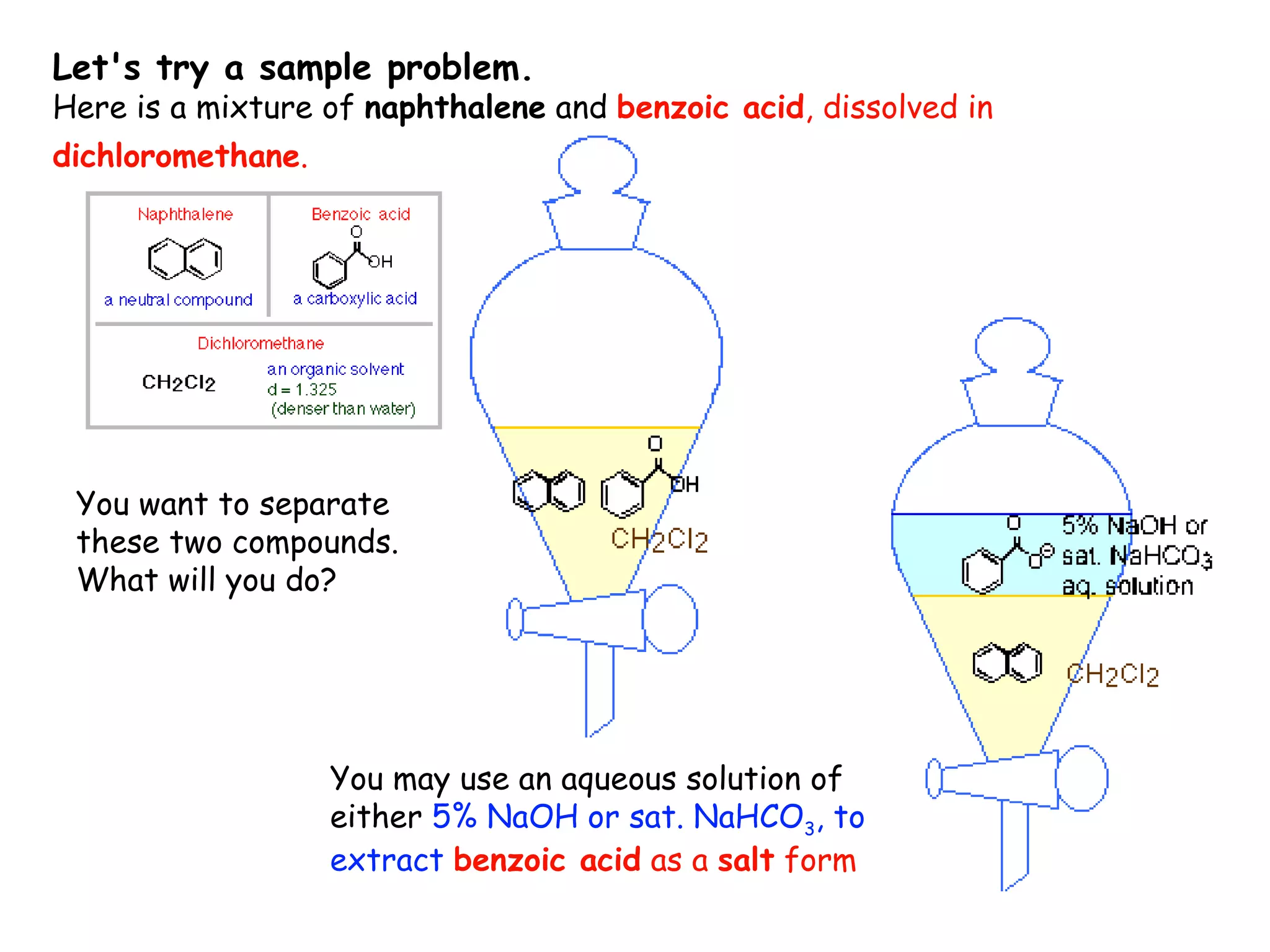
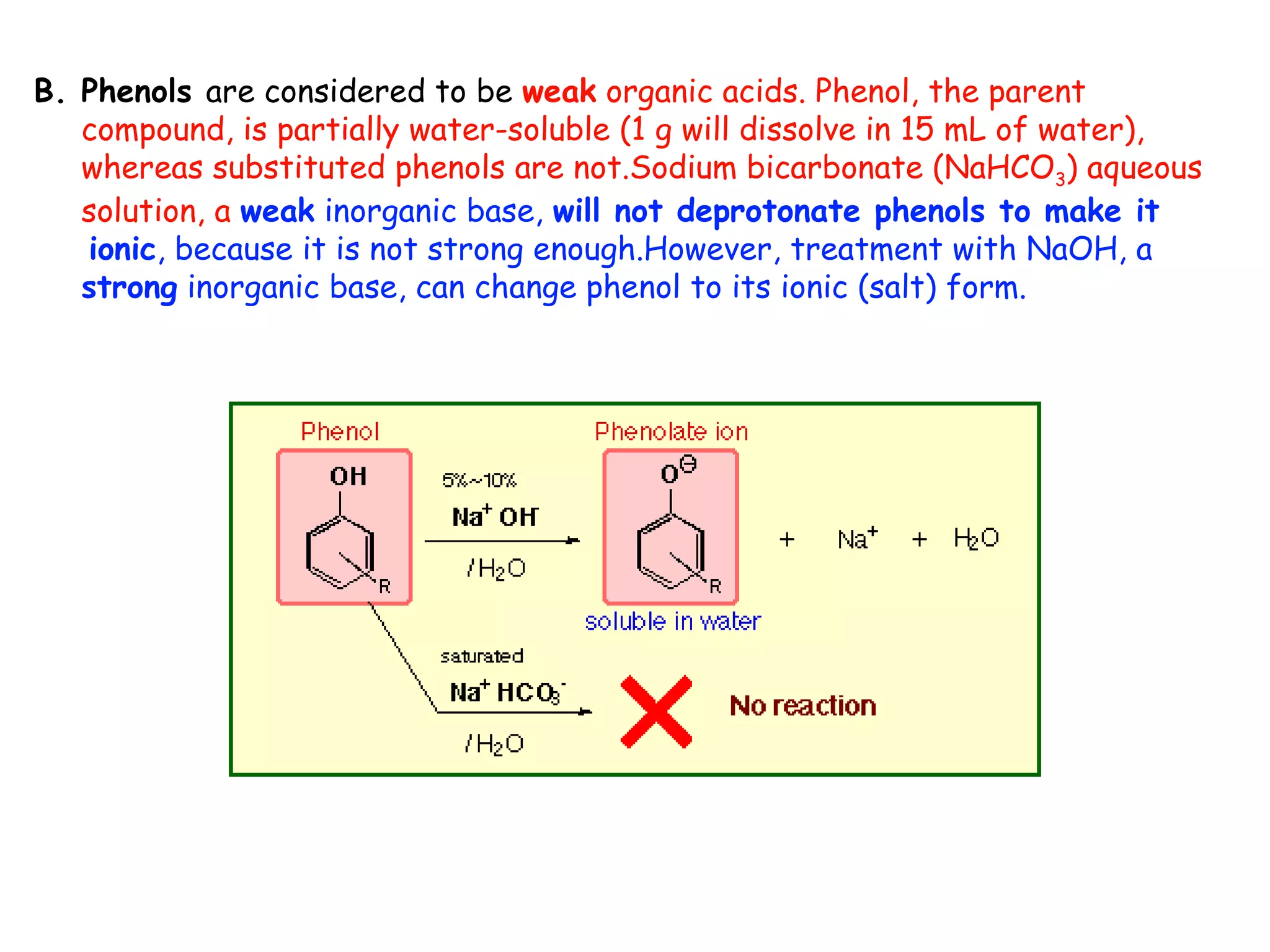
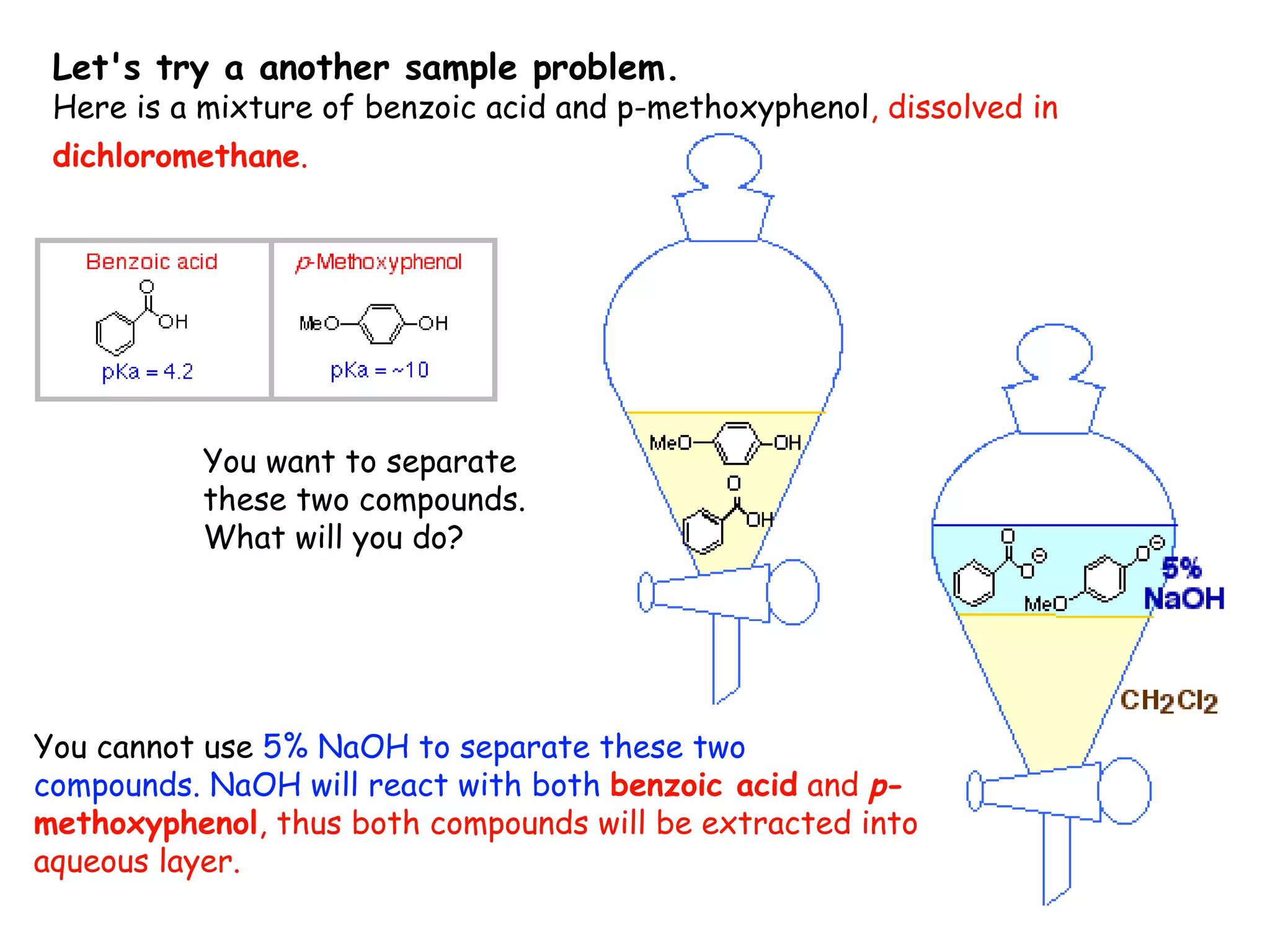
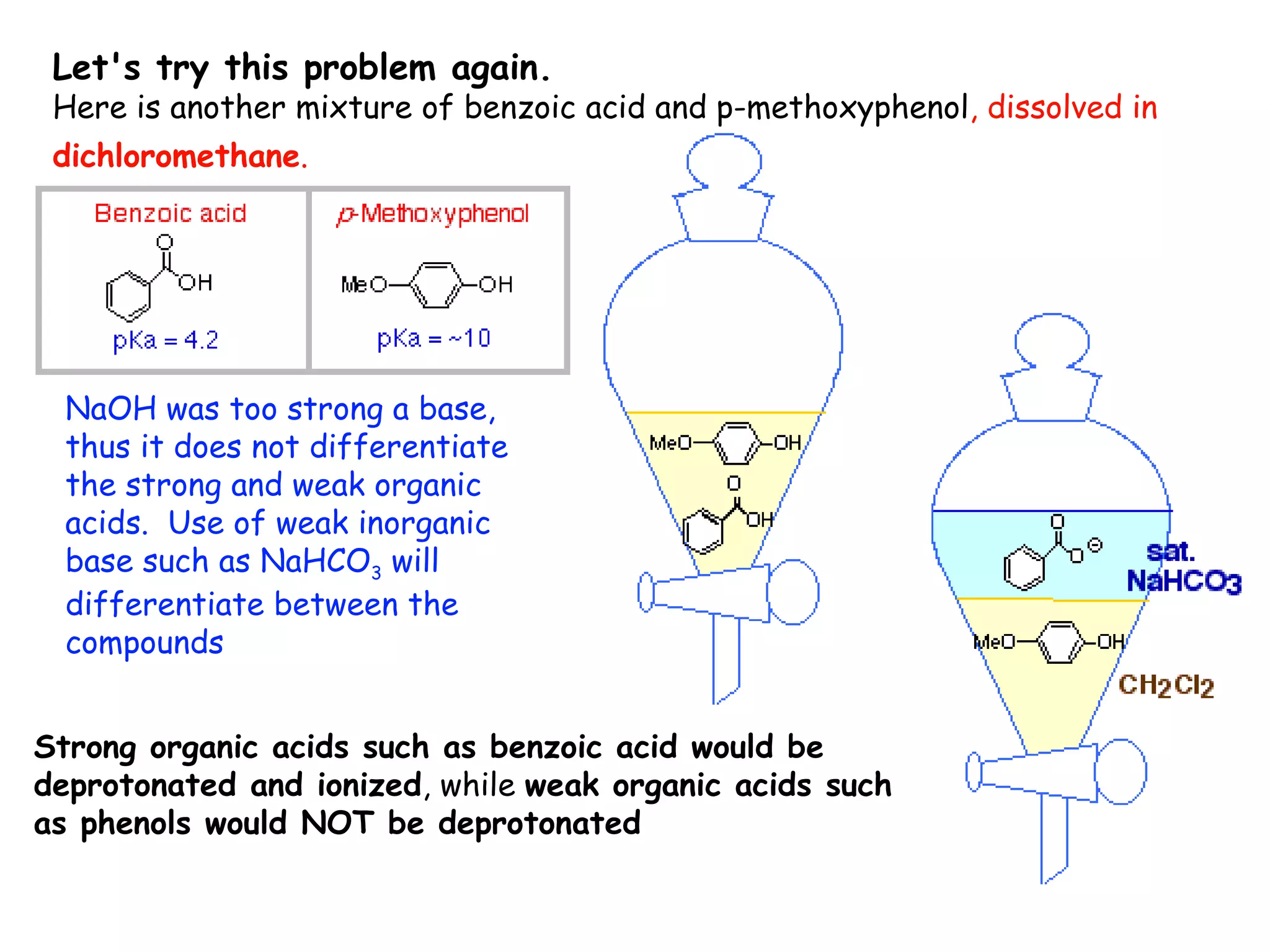
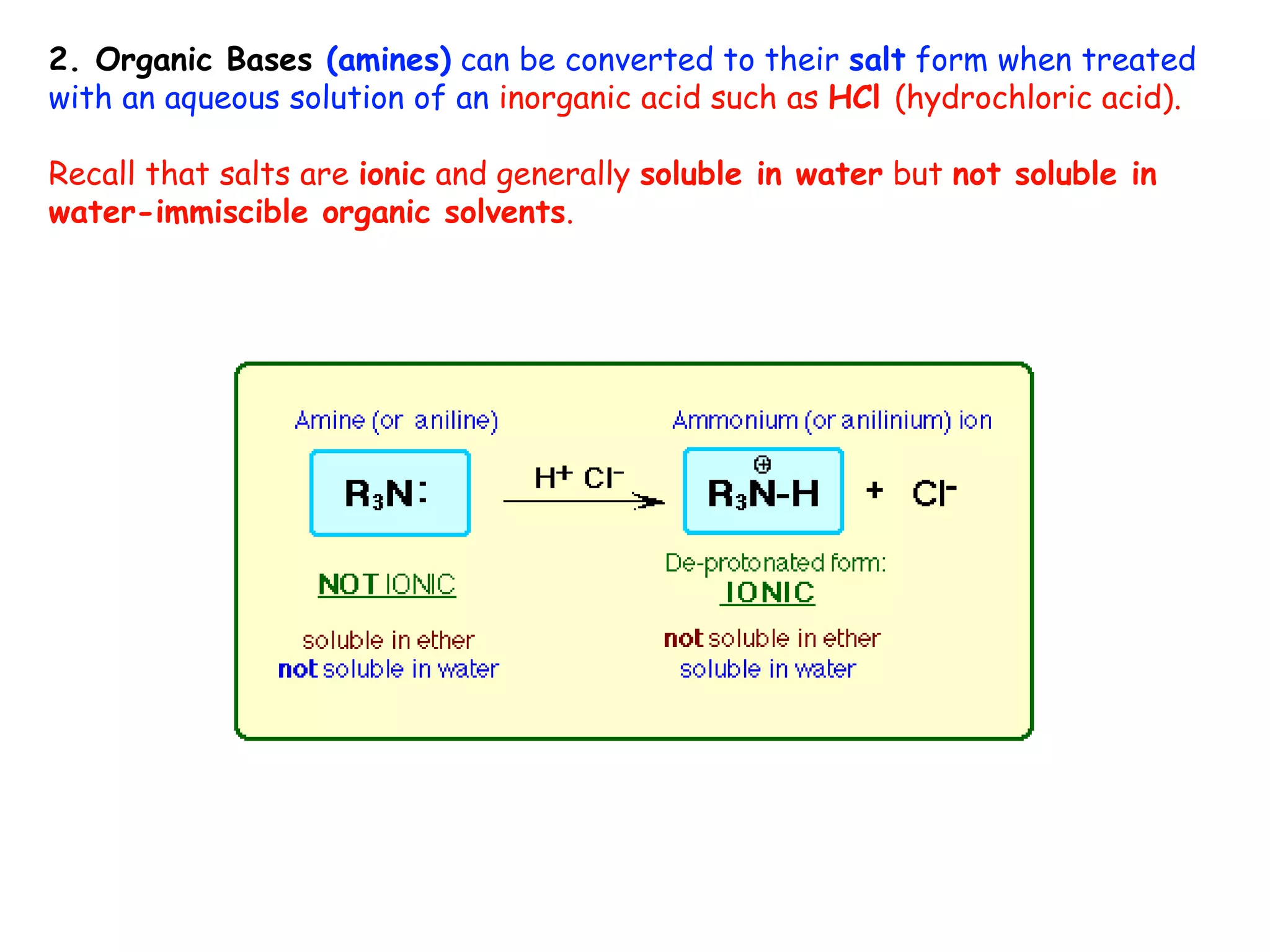
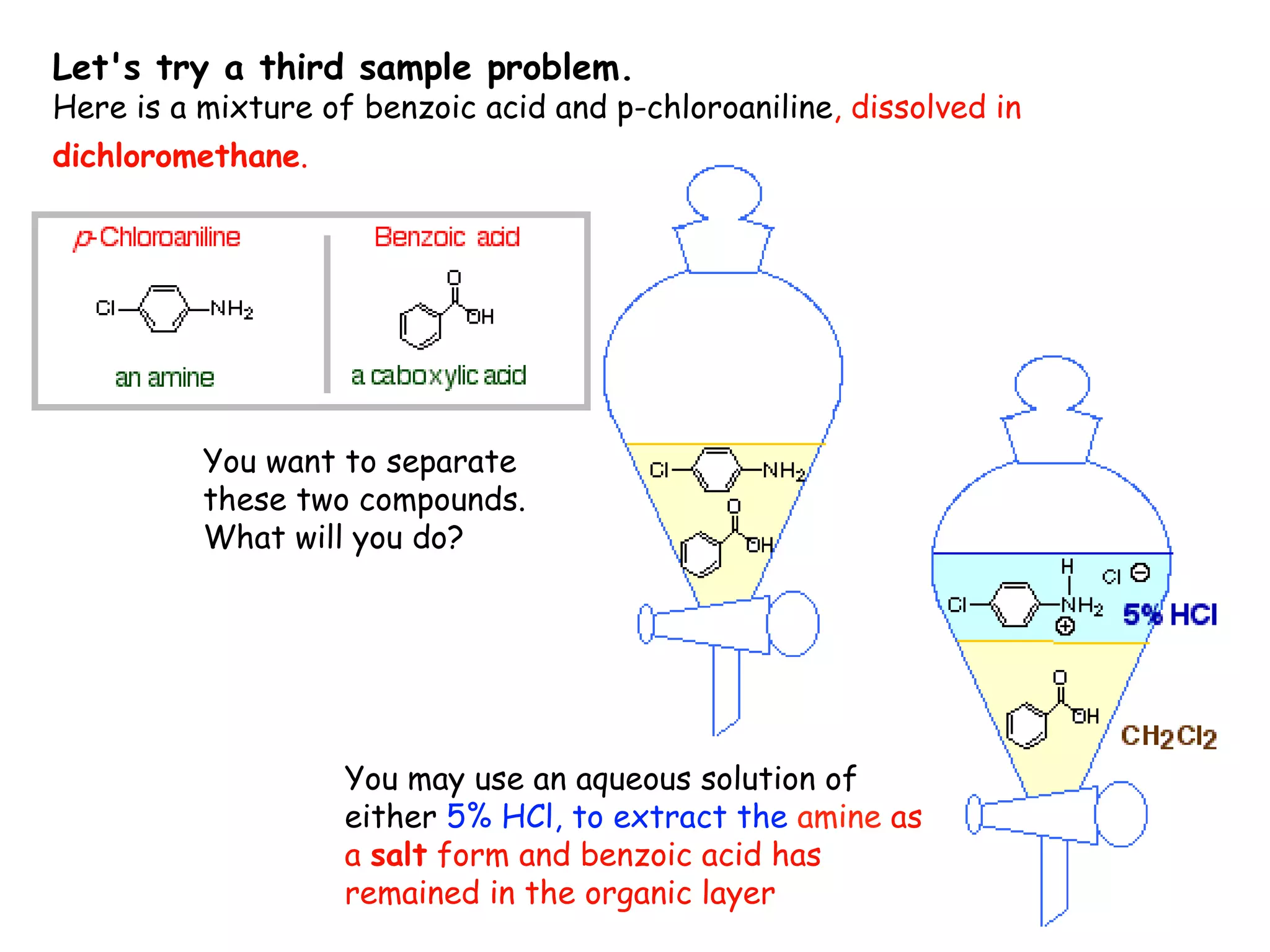
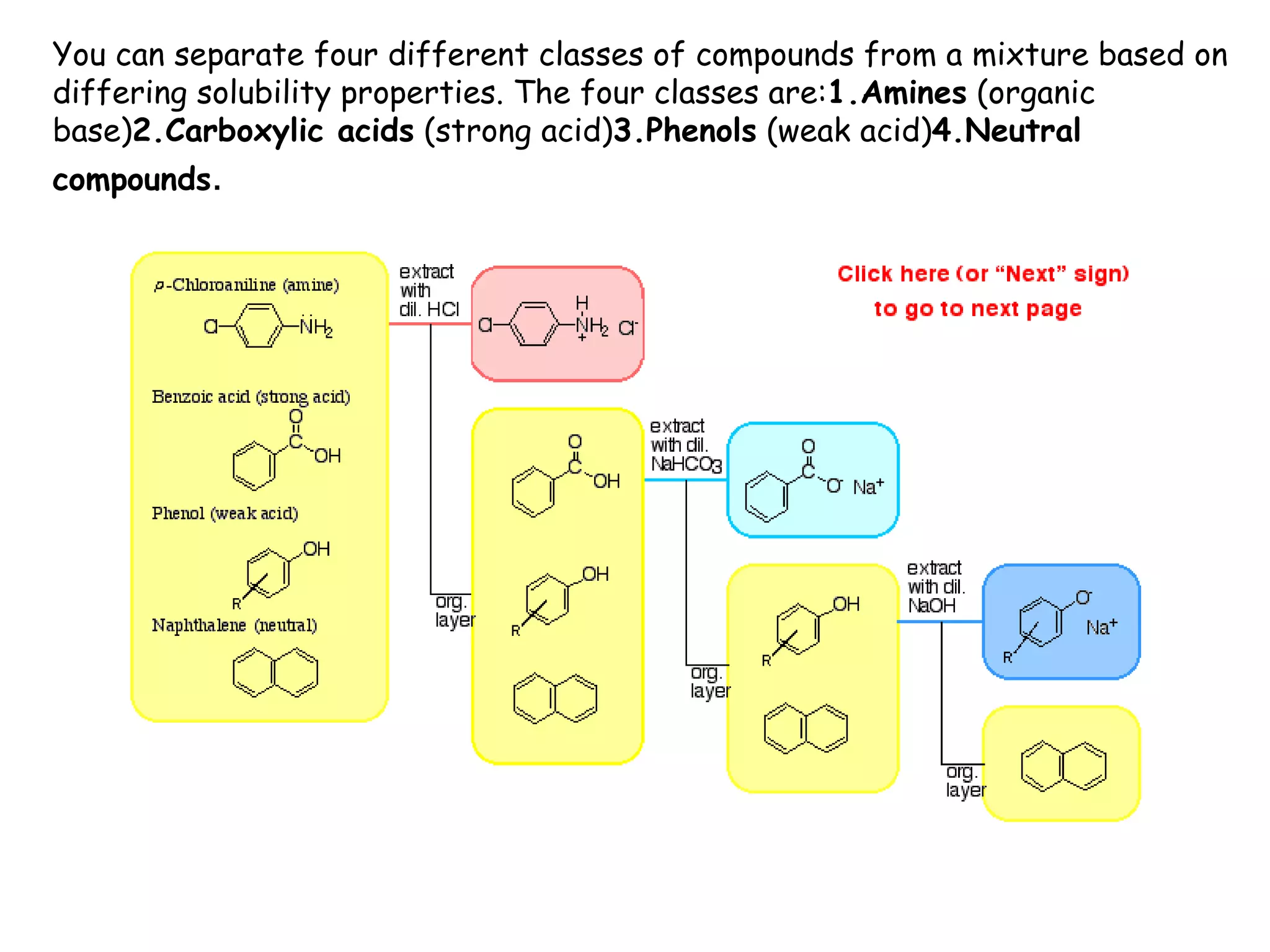
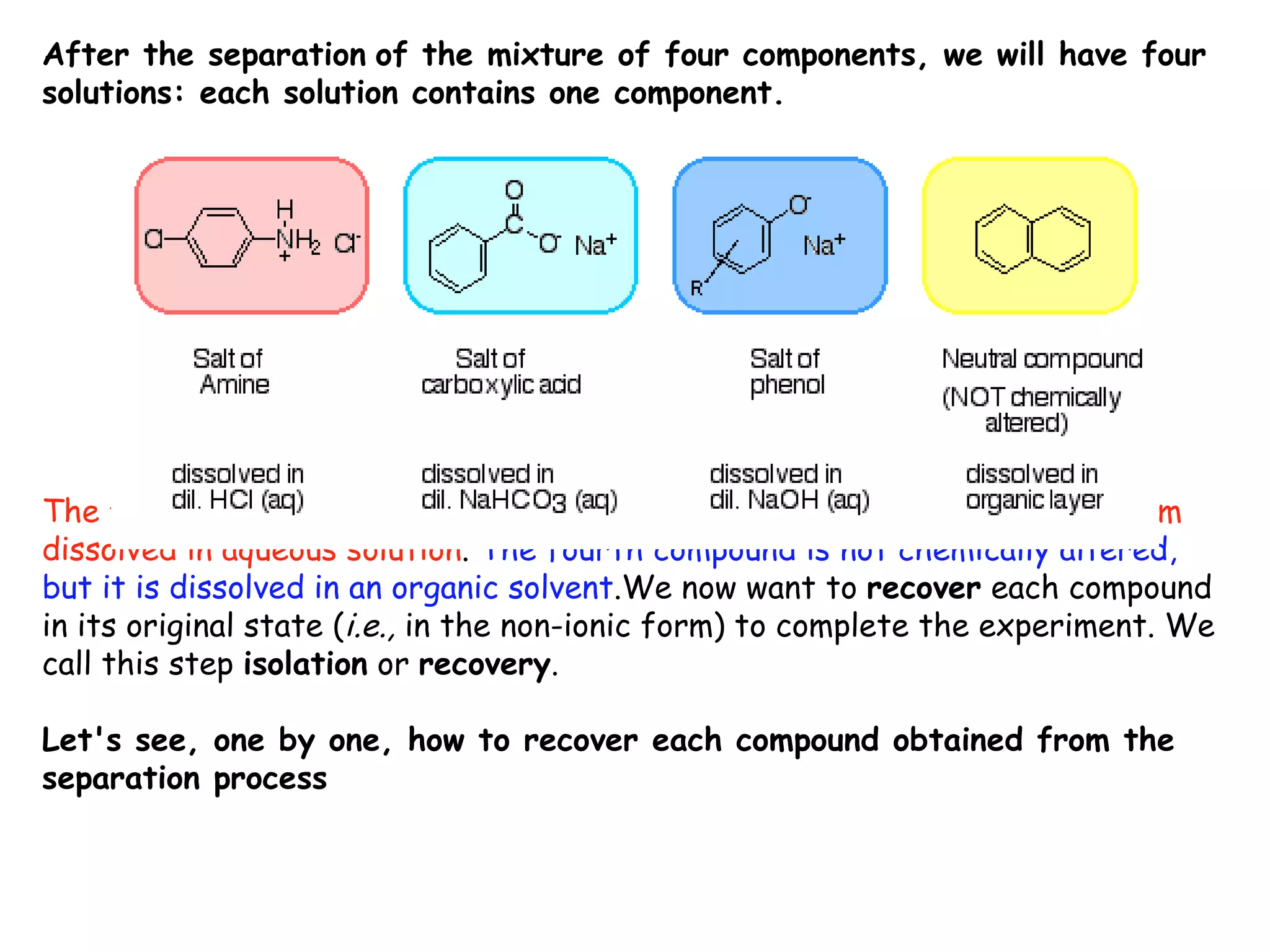
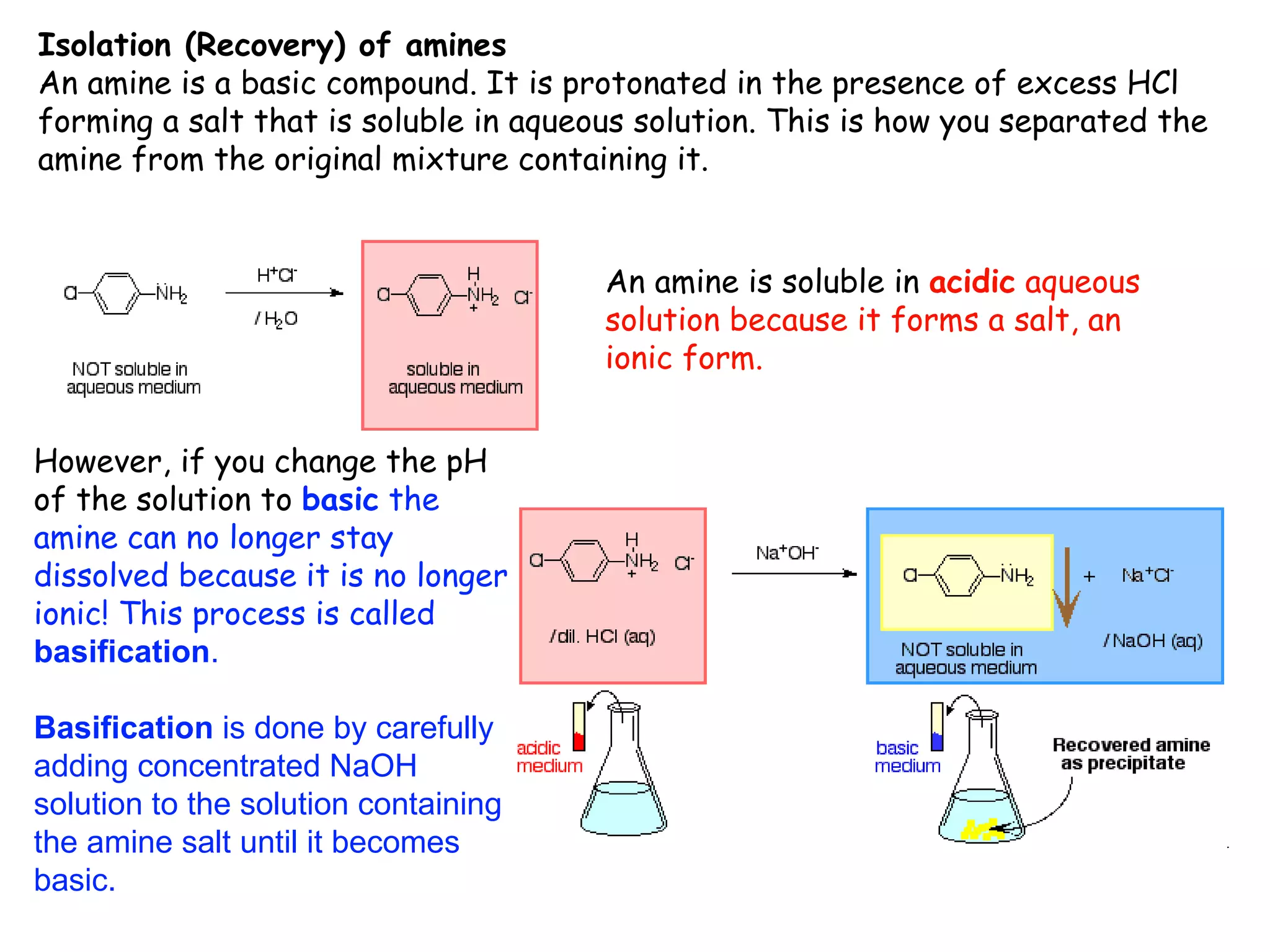
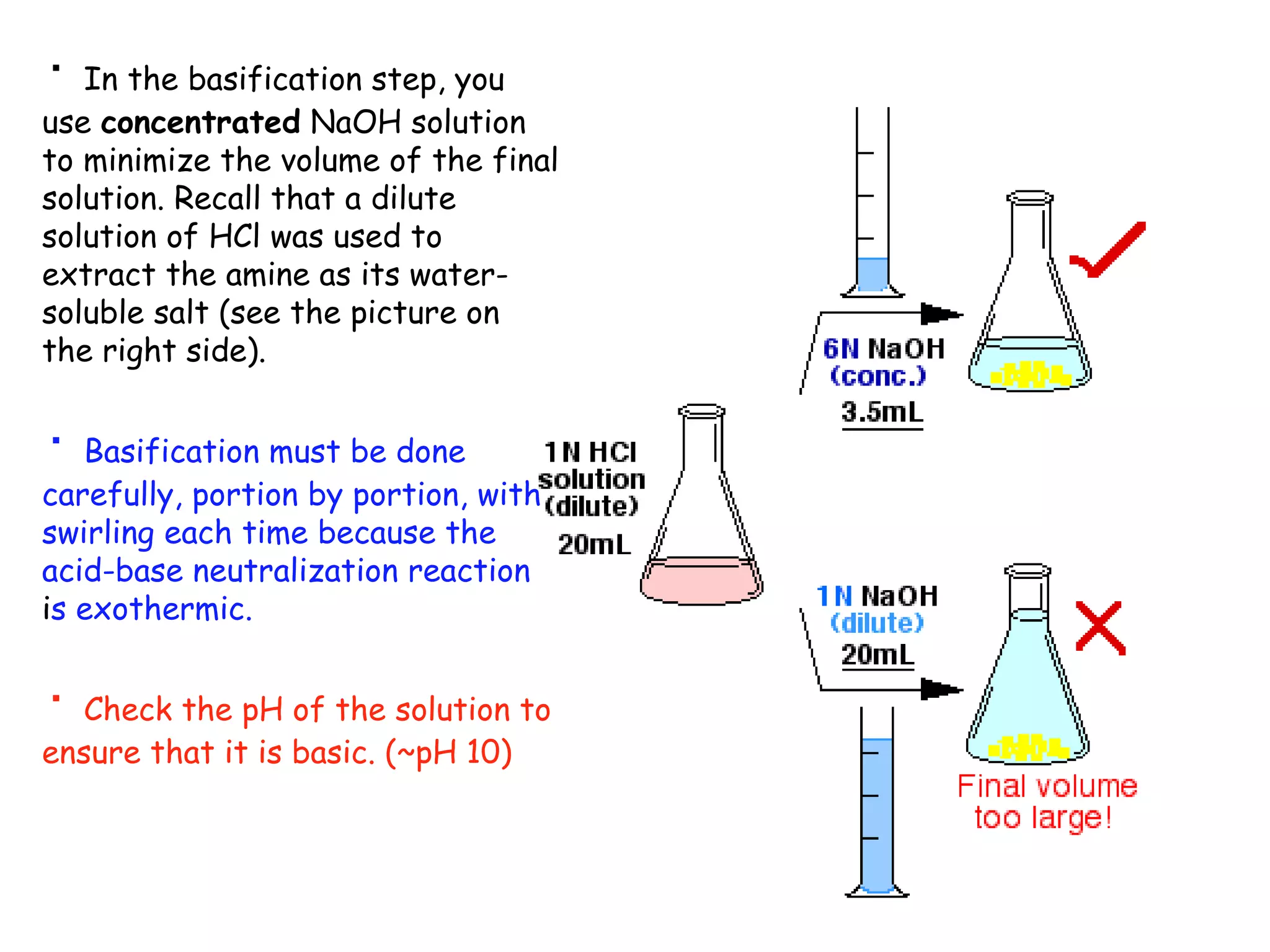
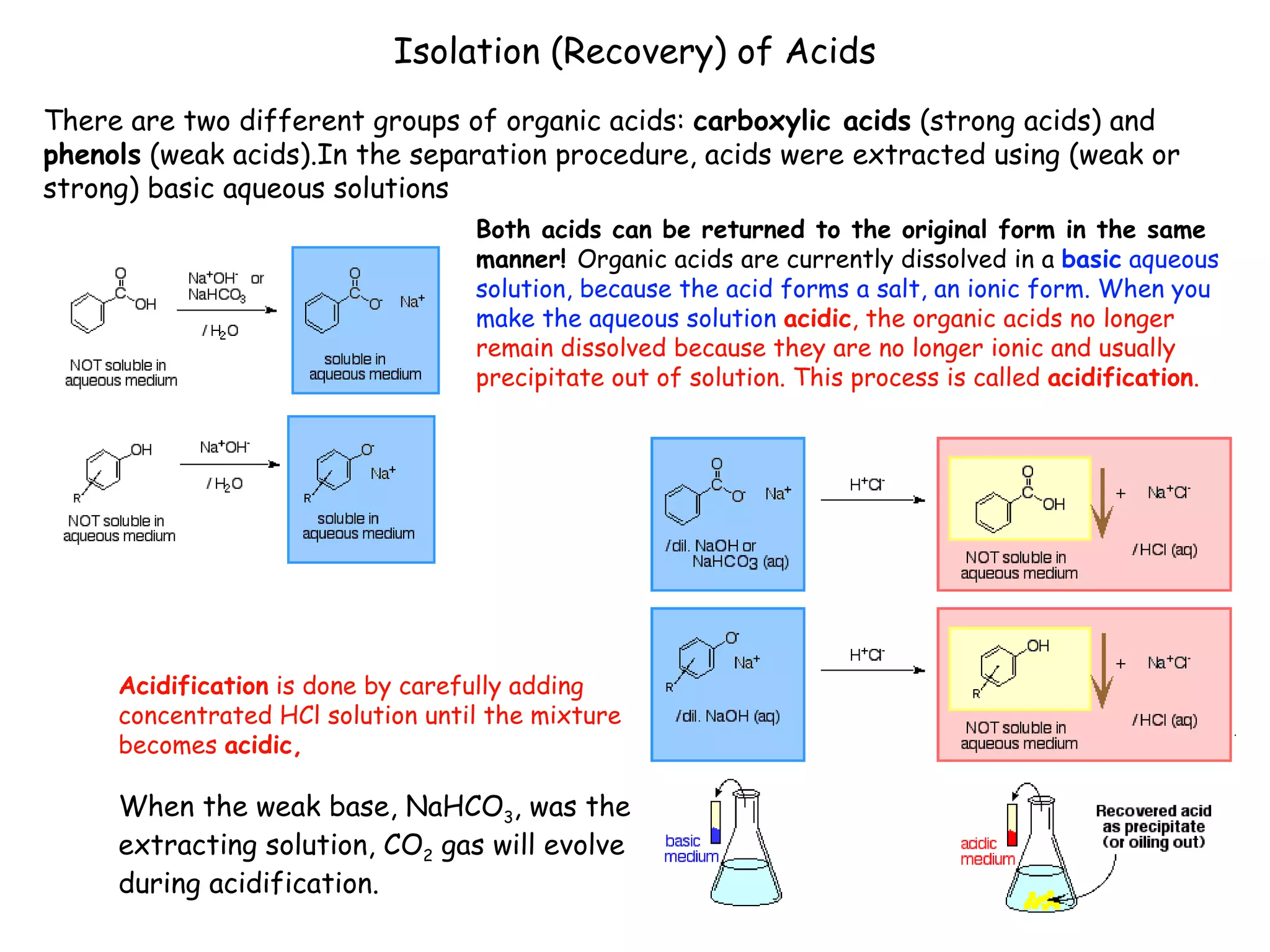
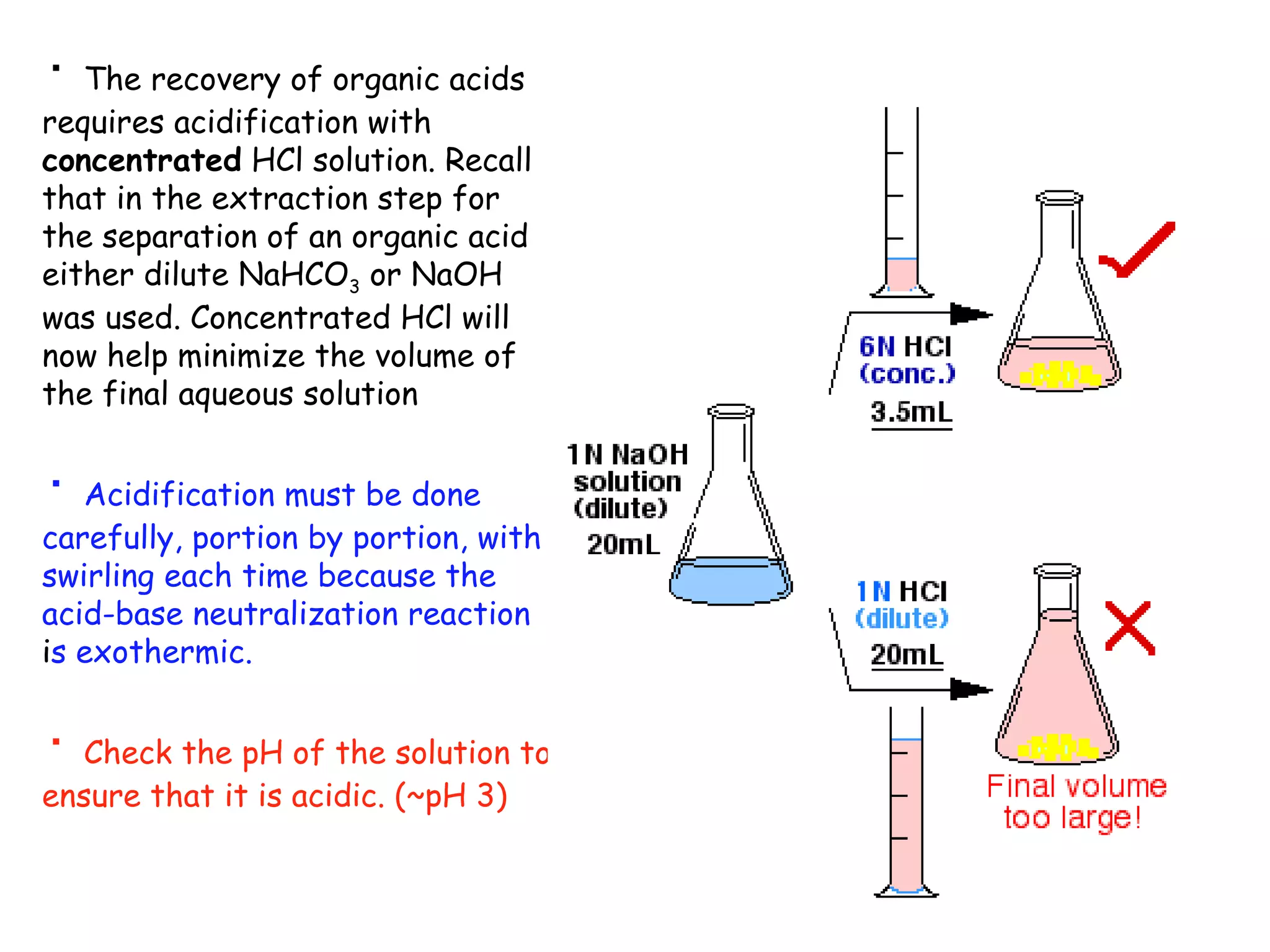
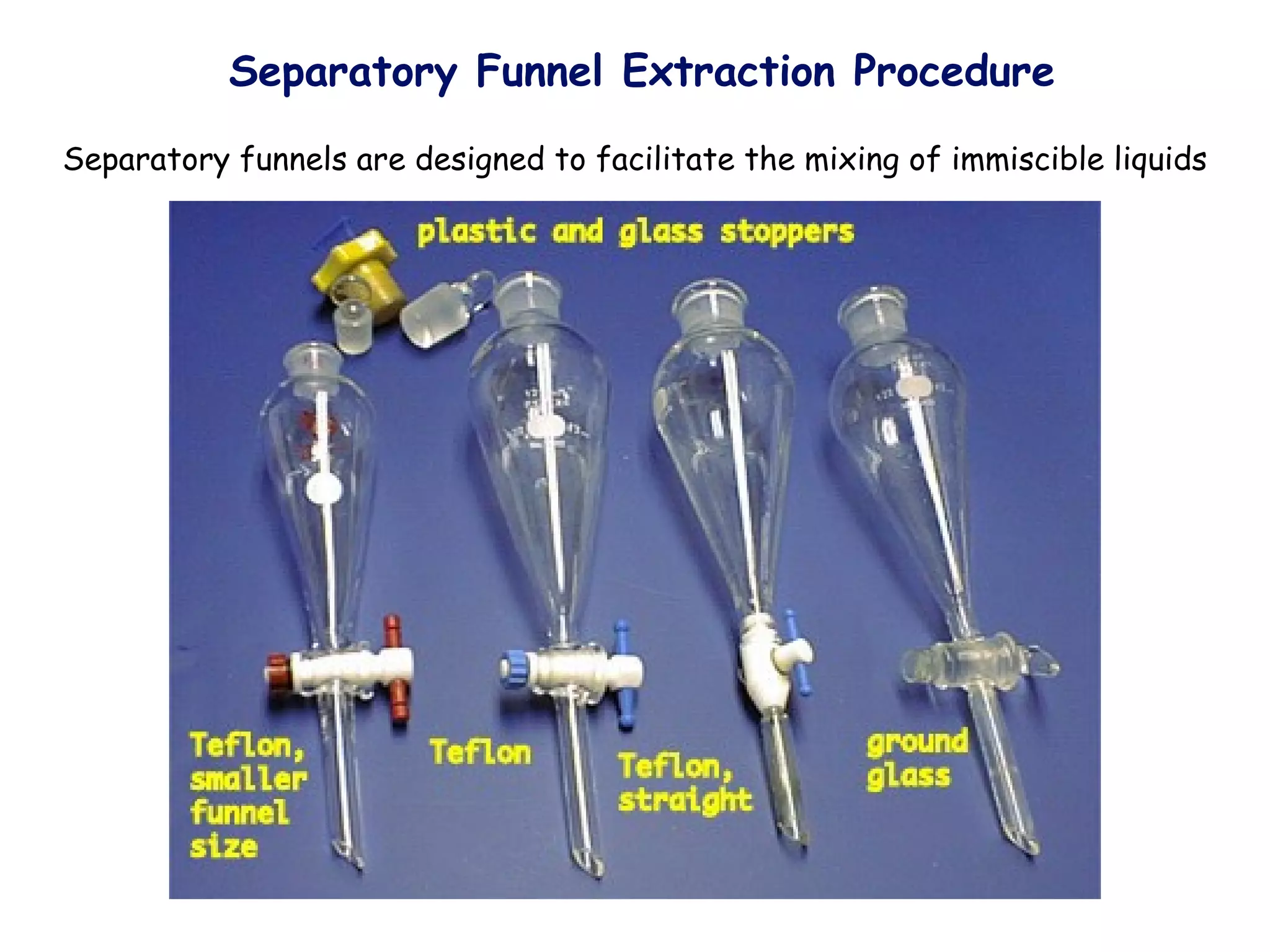
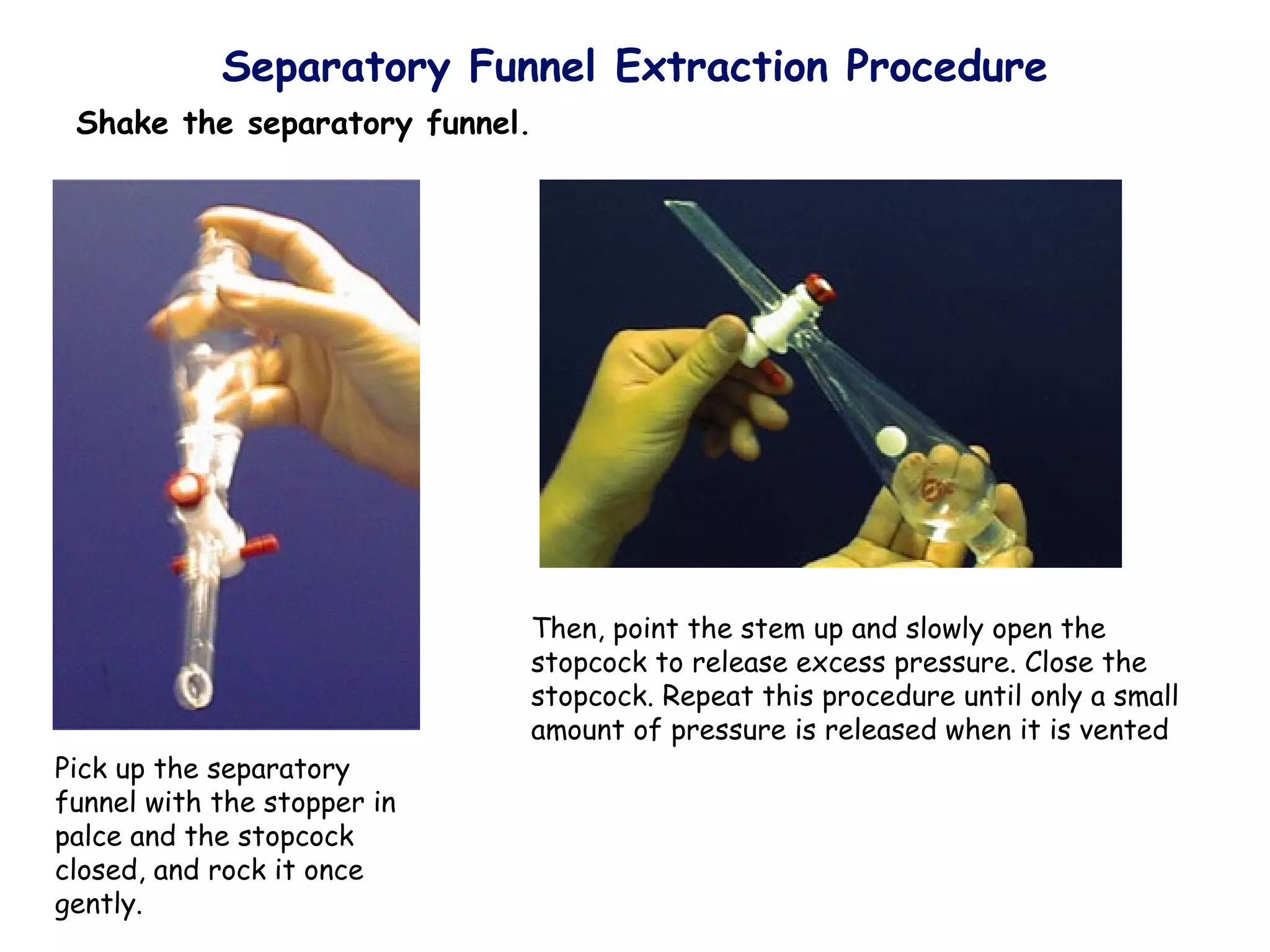
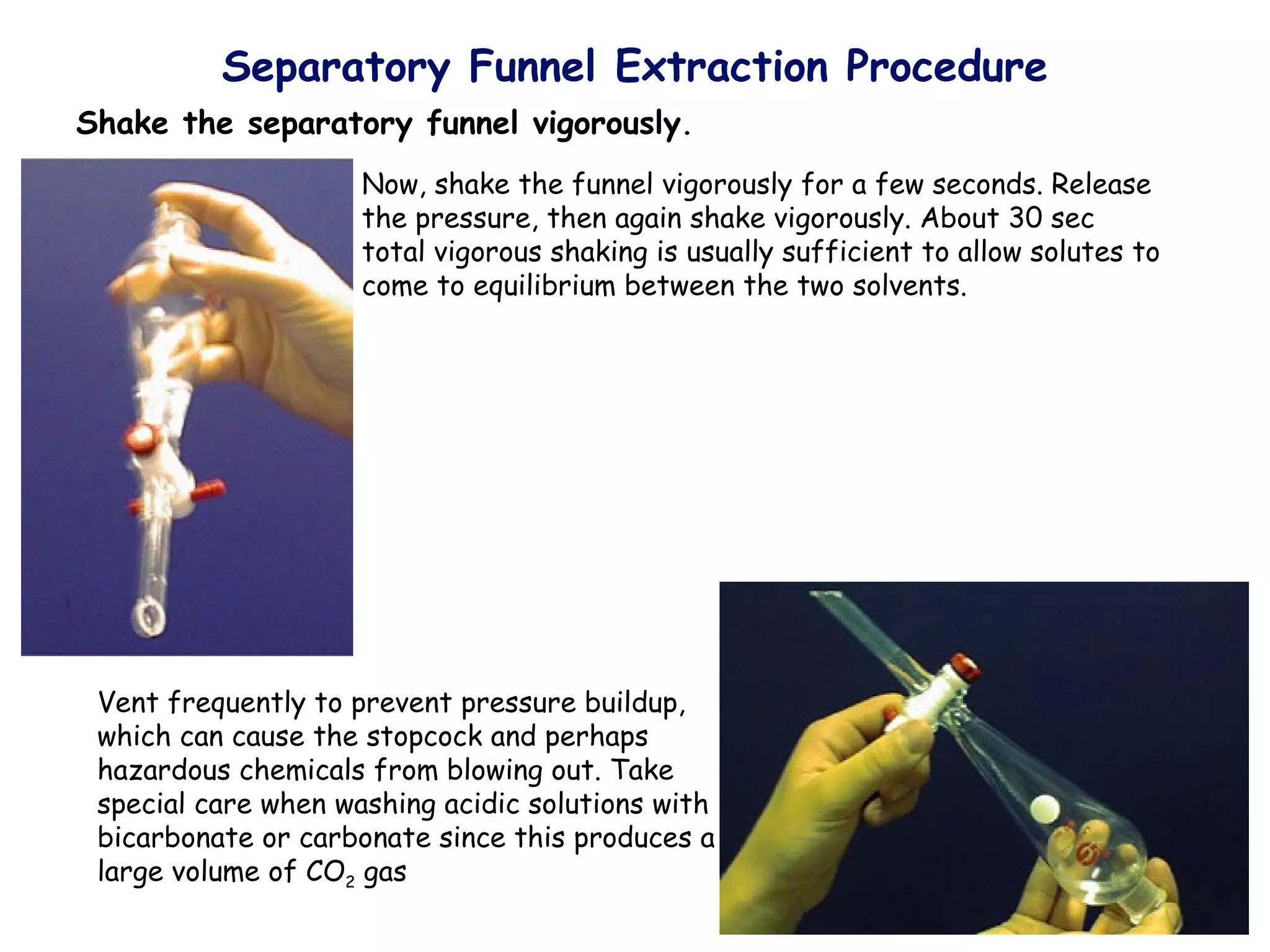
Liquid-liquid extraction is a technique used to separate components of a mixture based on differences in solubility. In this technique, a solvent is used to transfer a solute from one liquid phase to another immiscible liquid phase. For example, sugar can be extracted from a vegetable oil and water mixture by shaking the mixture, as the sugar is more soluble in water. The success of liquid-liquid extraction depends on choosing an extraction solvent that exploits differences in a compound's solubility between solvents. Additionally, compounds can be made more soluble in water by converting them to ionic salt forms using acid-base chemistry.