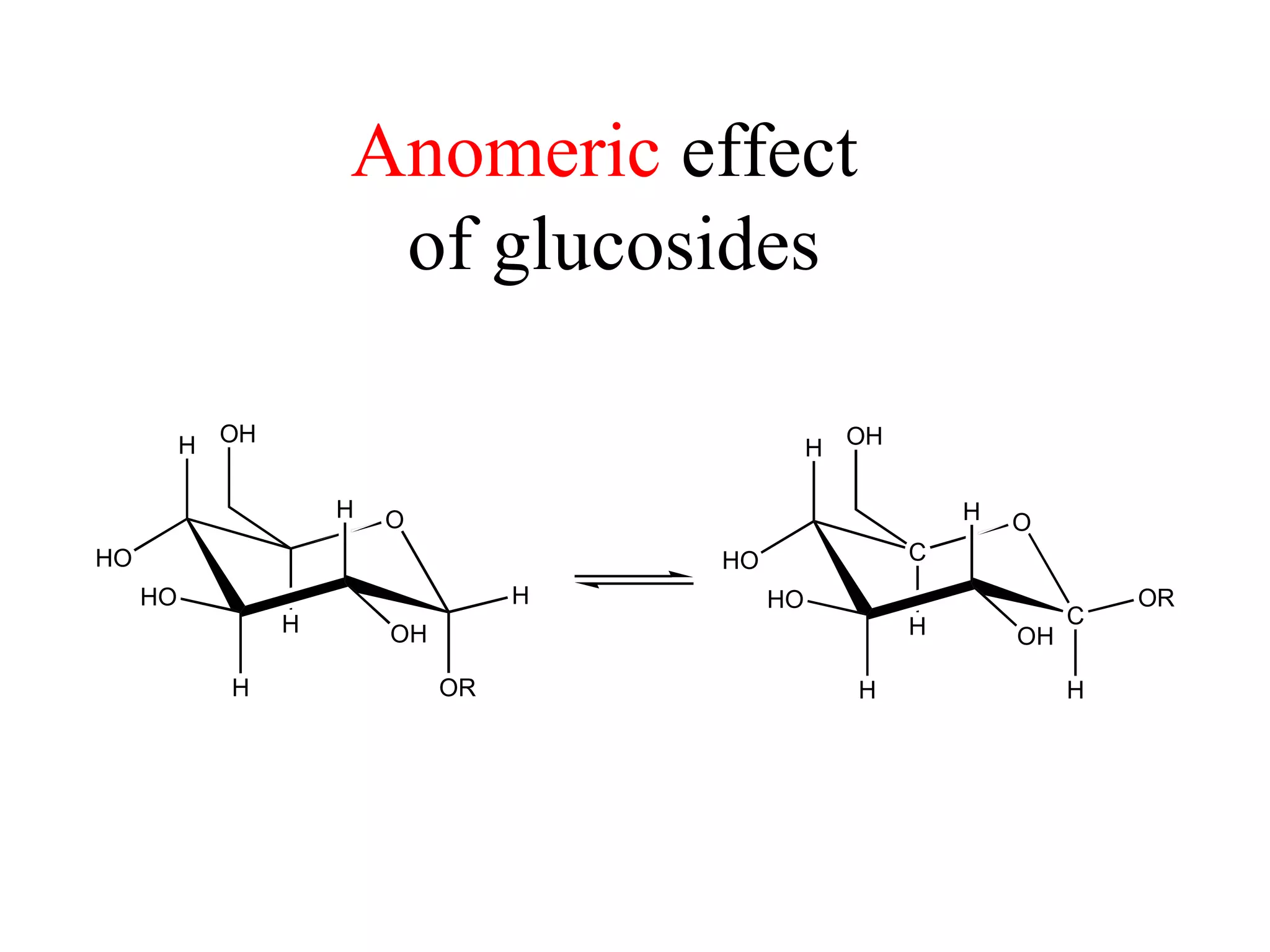
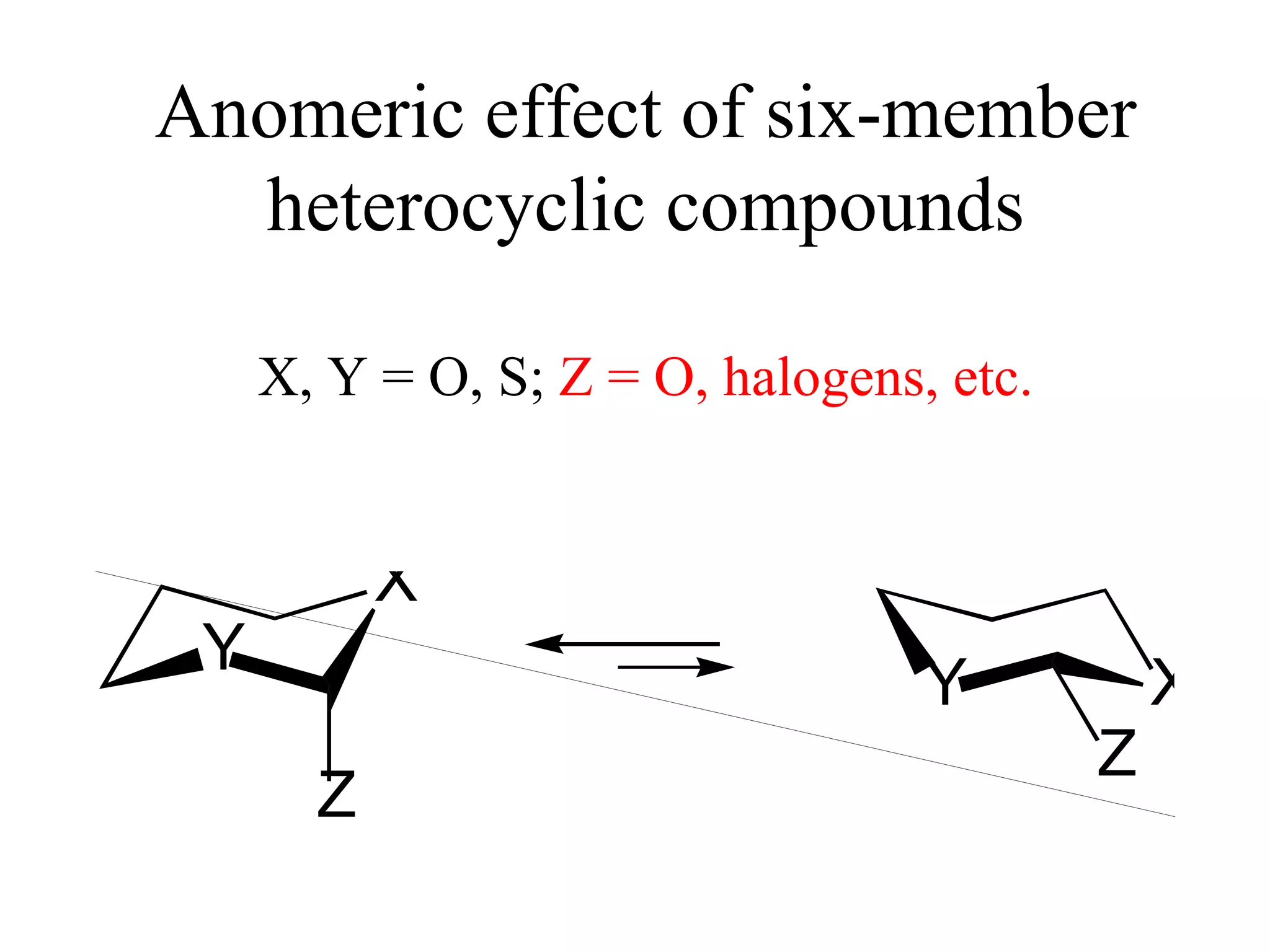
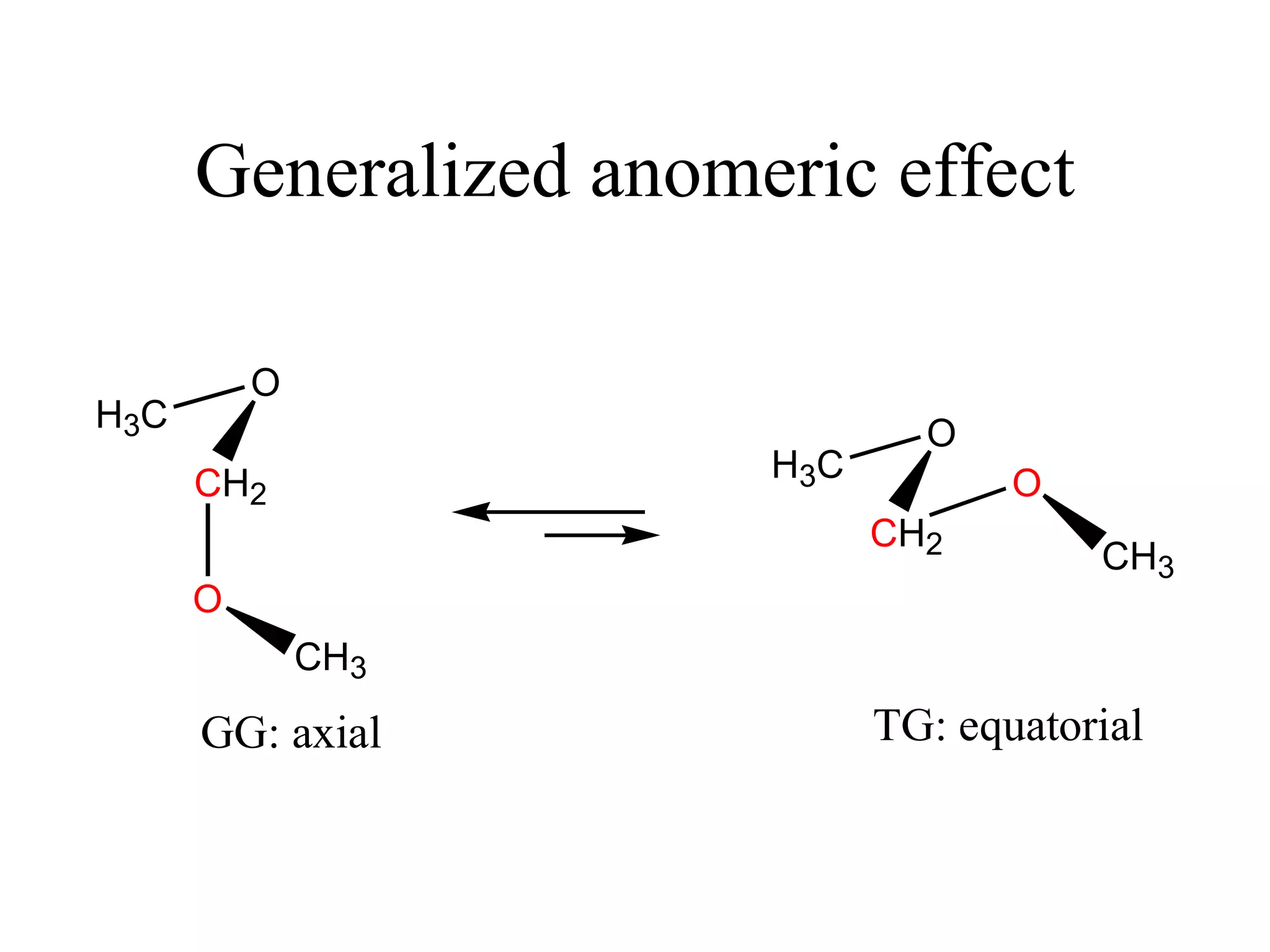
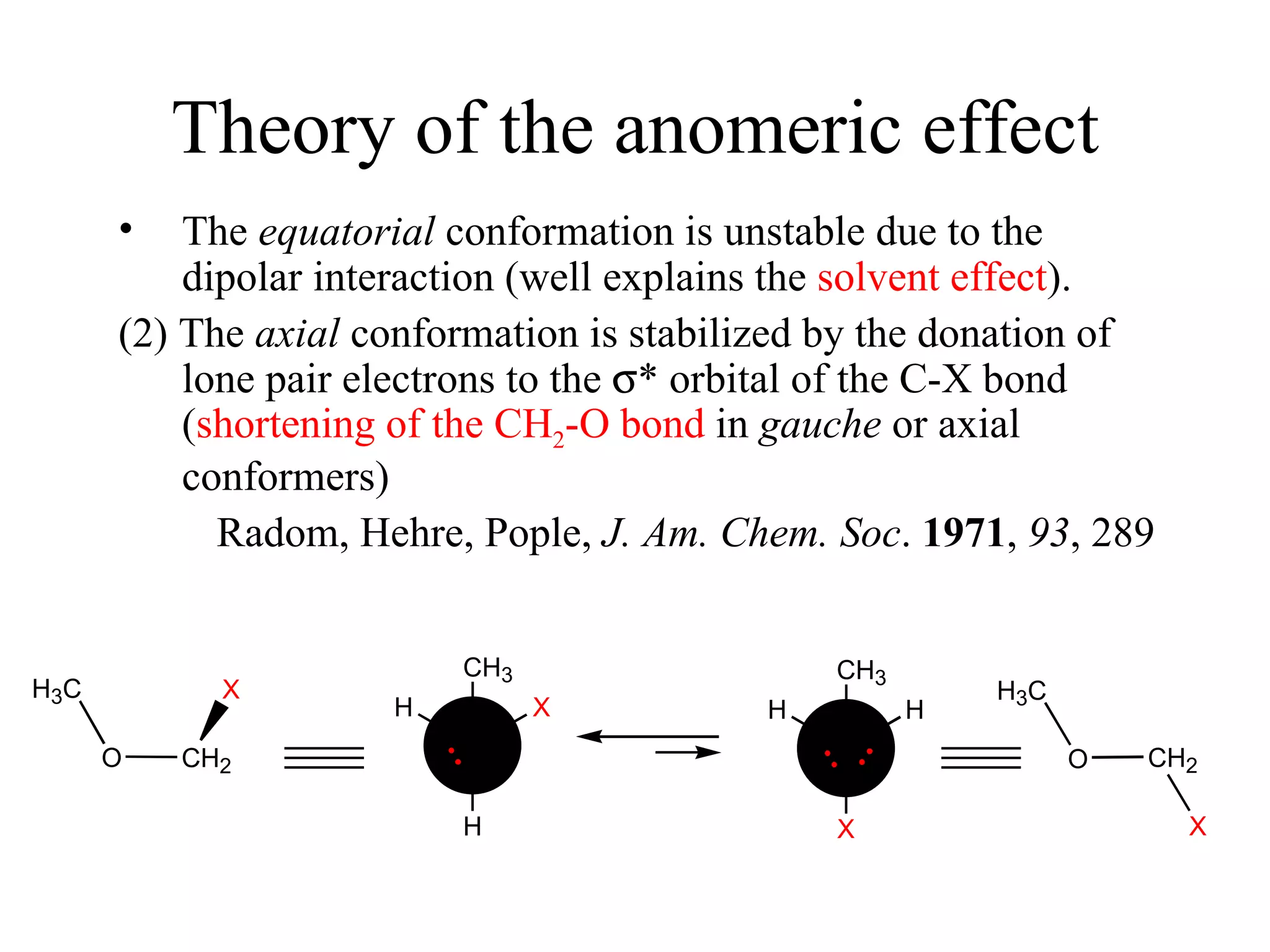
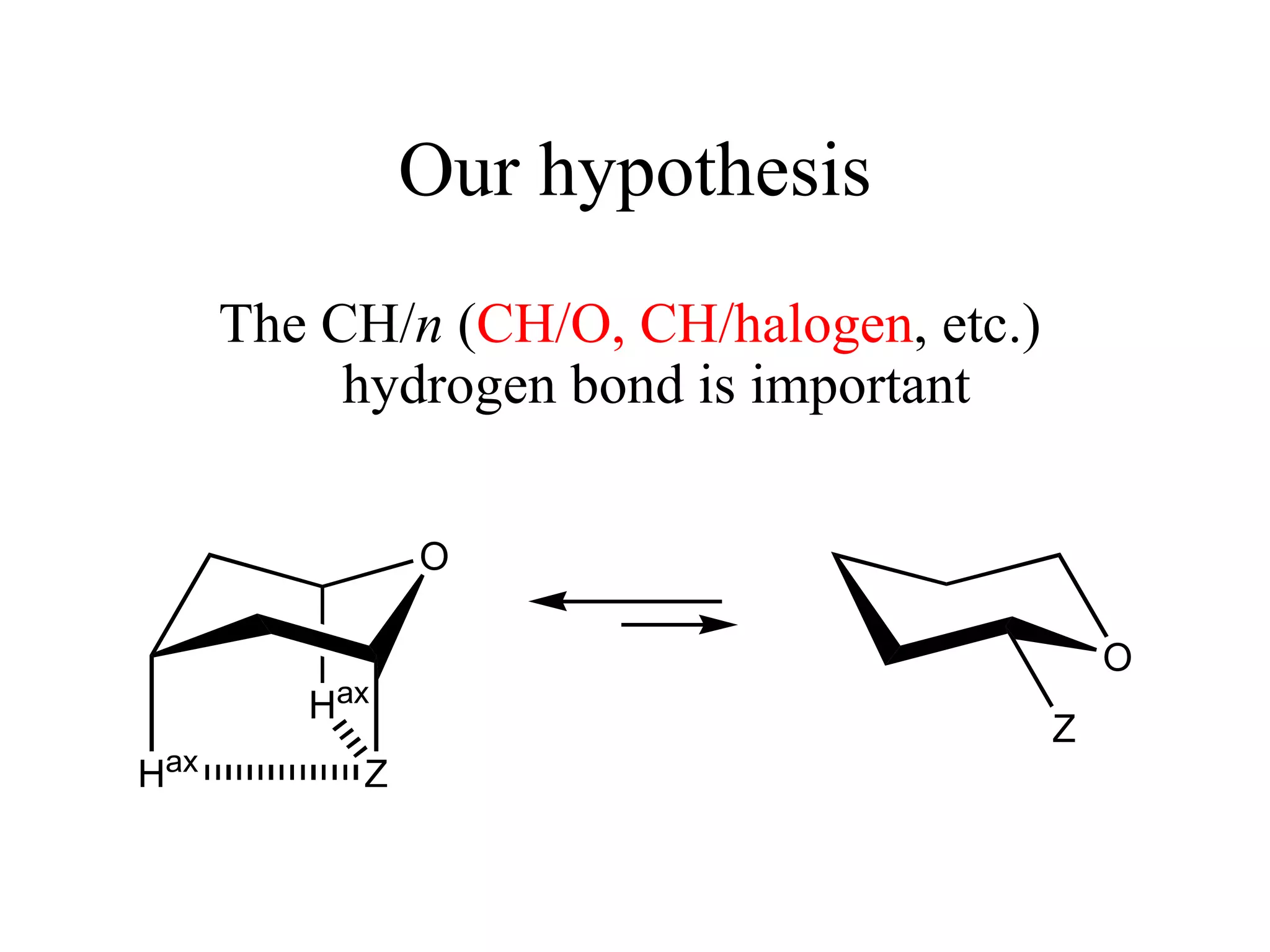
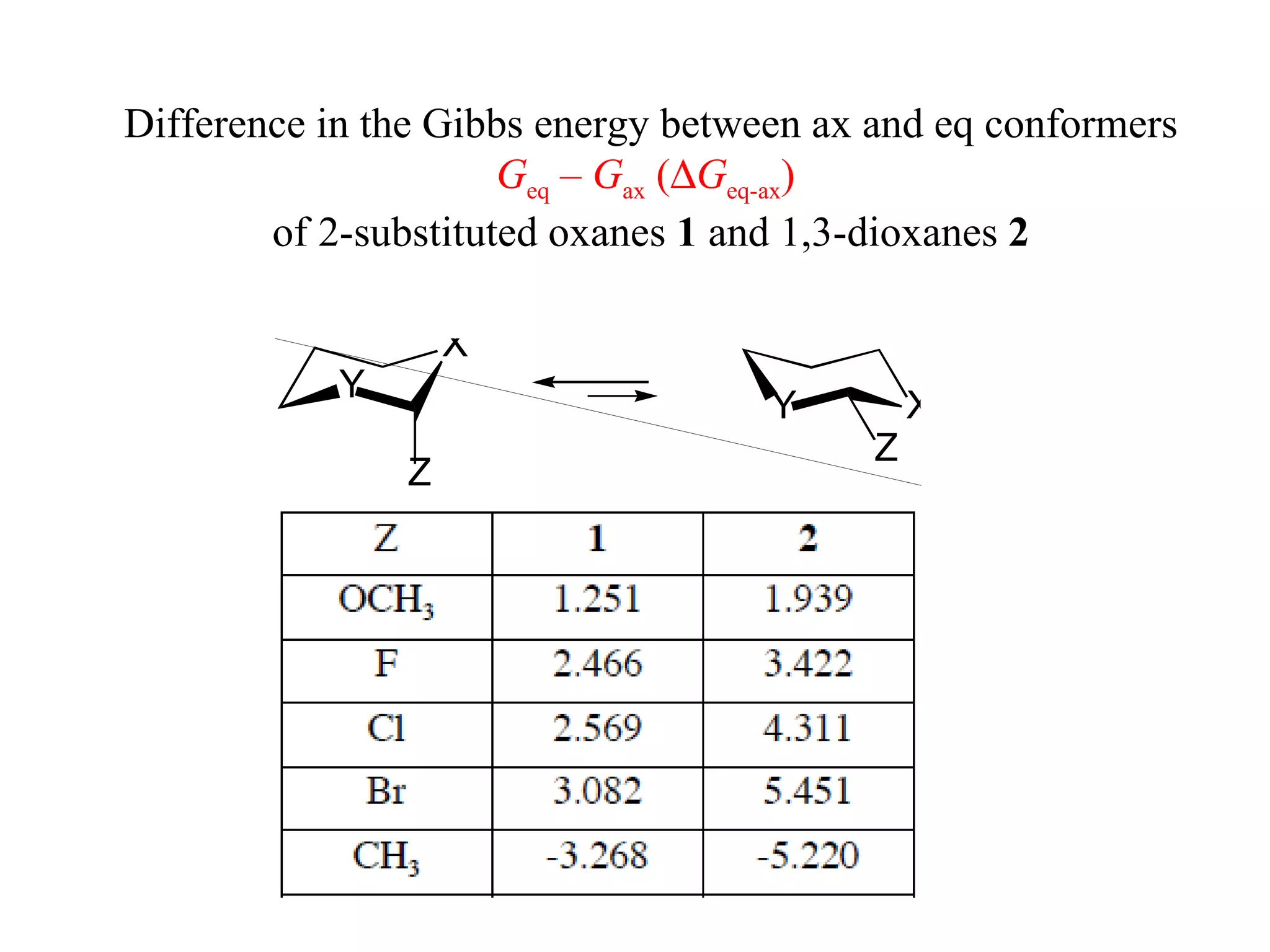
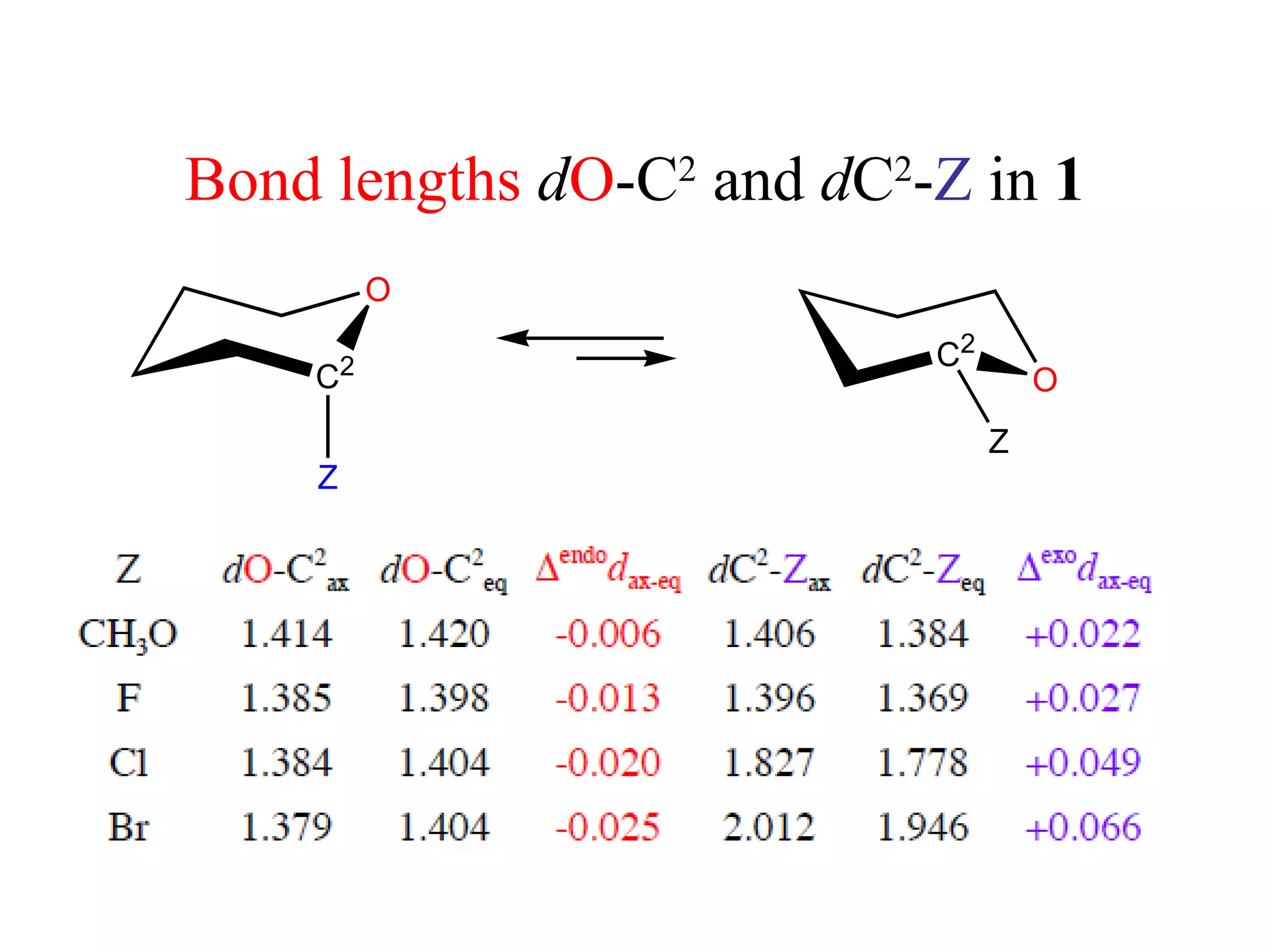
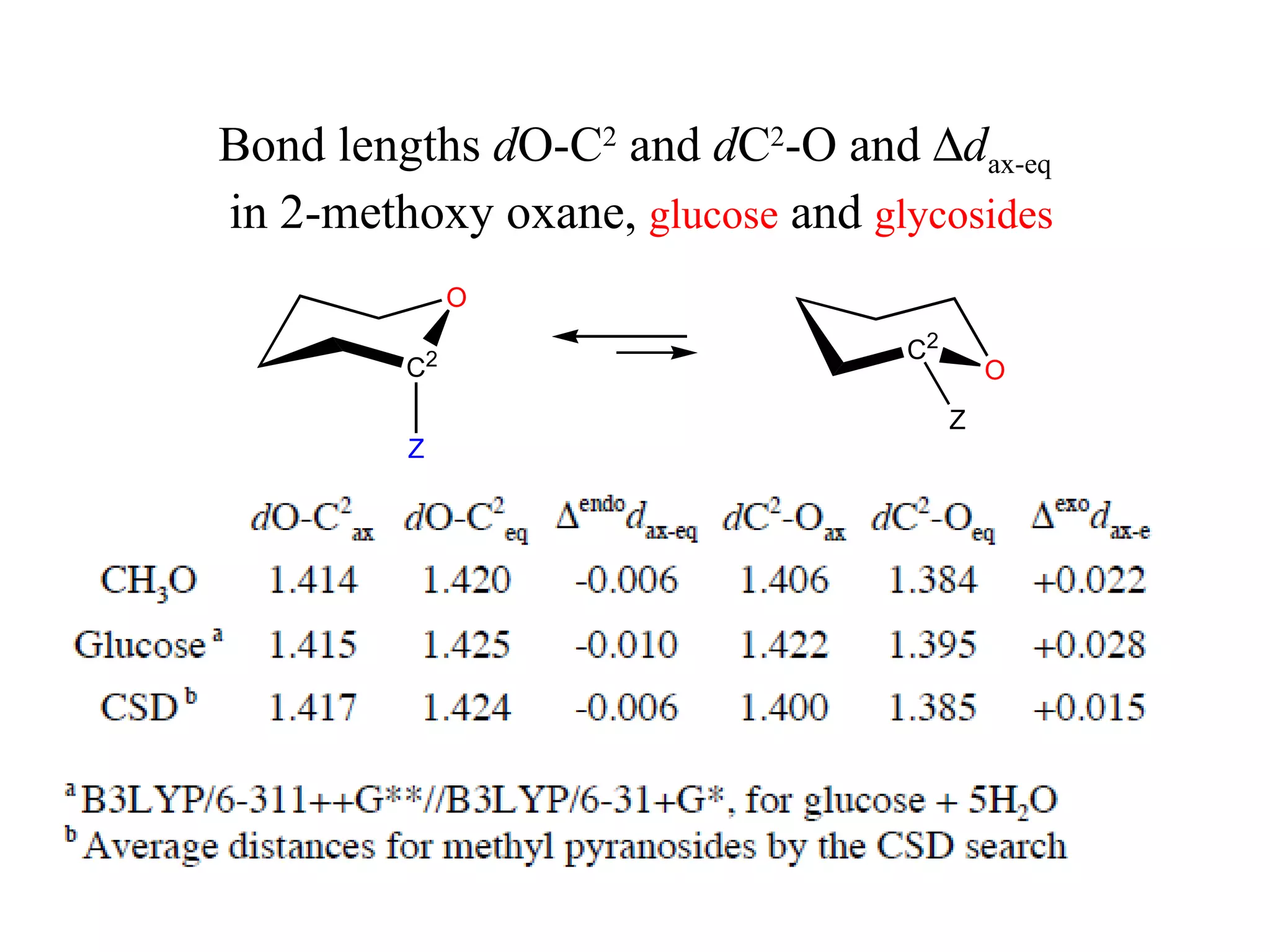
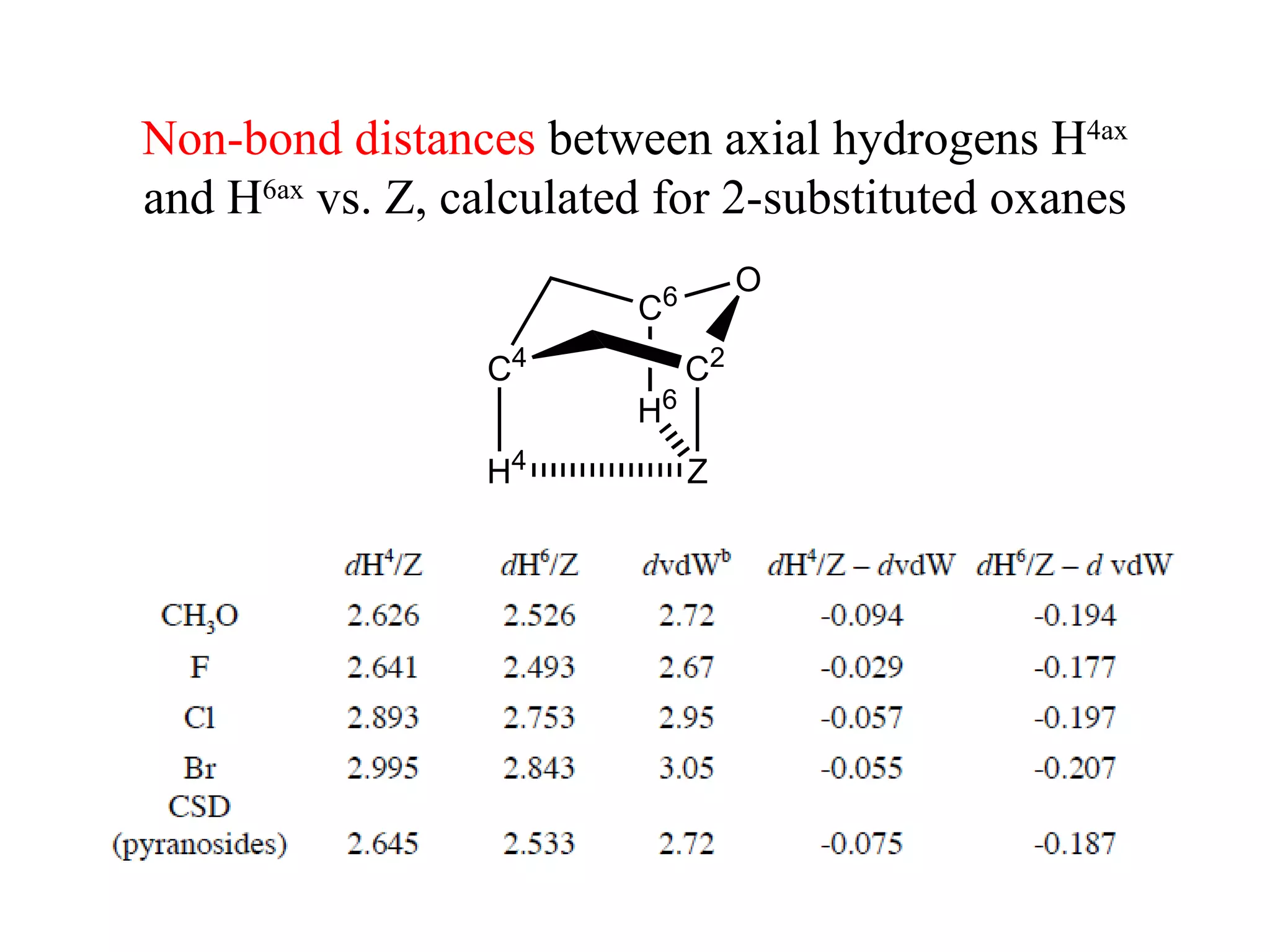
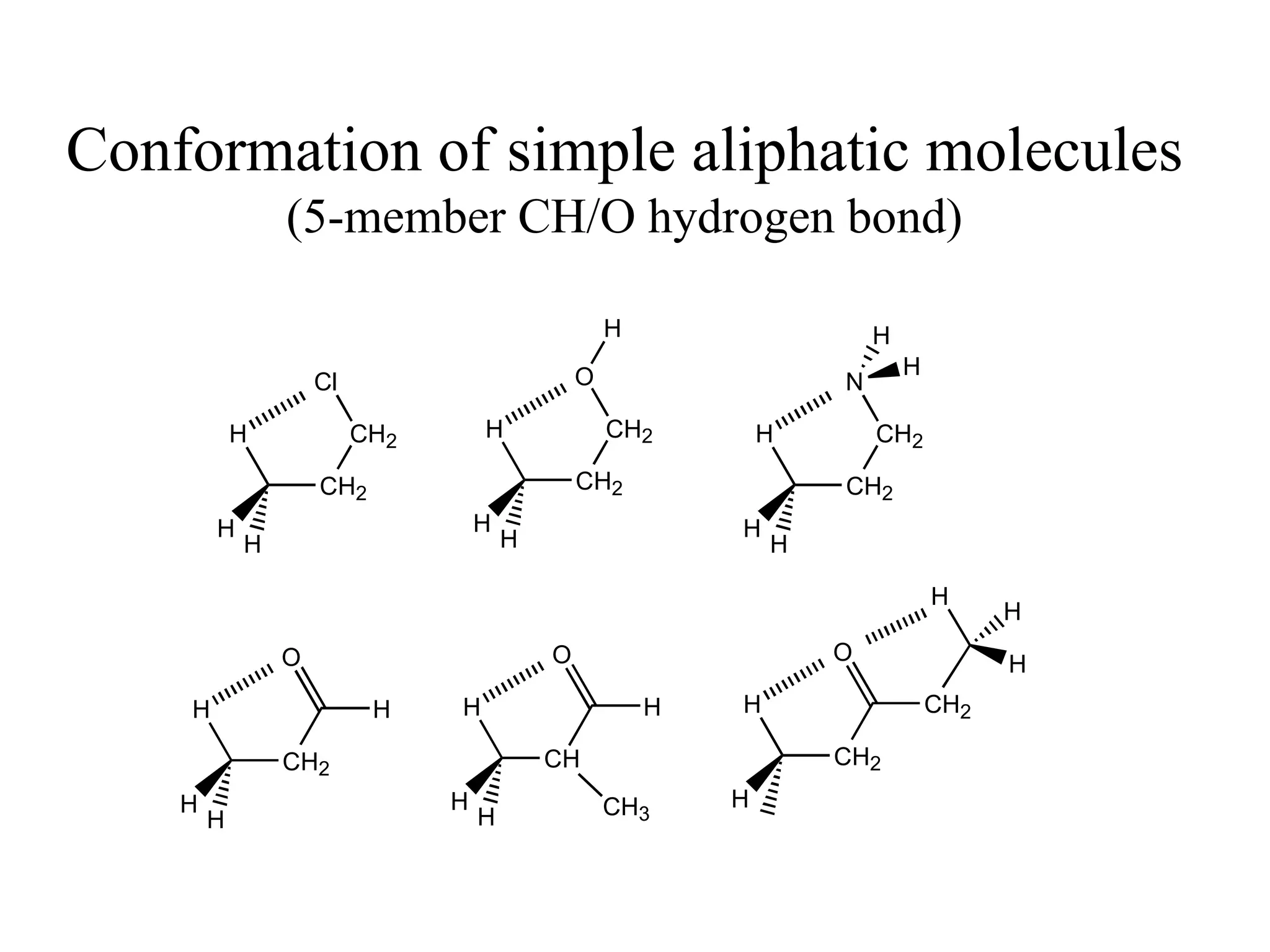
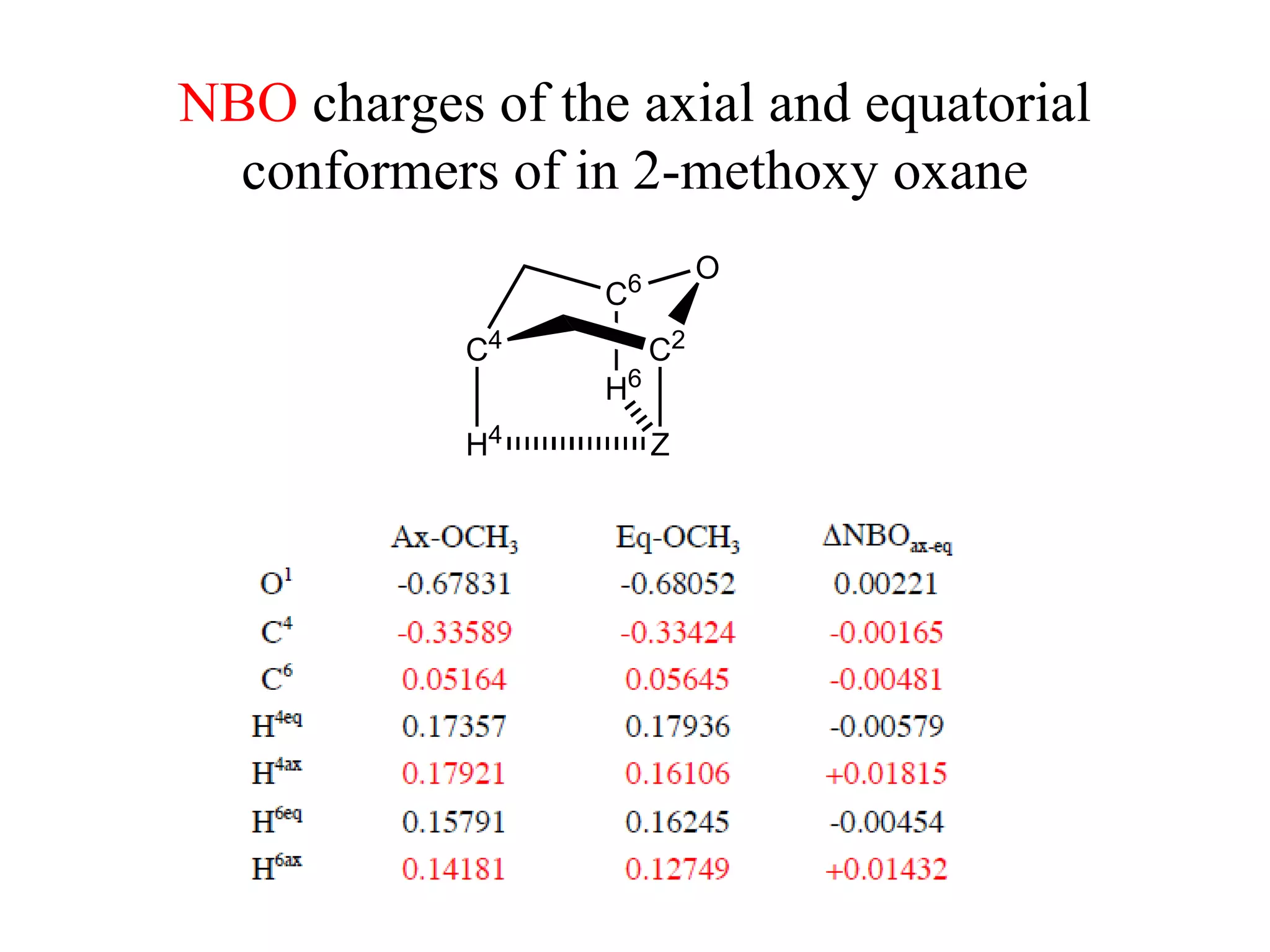
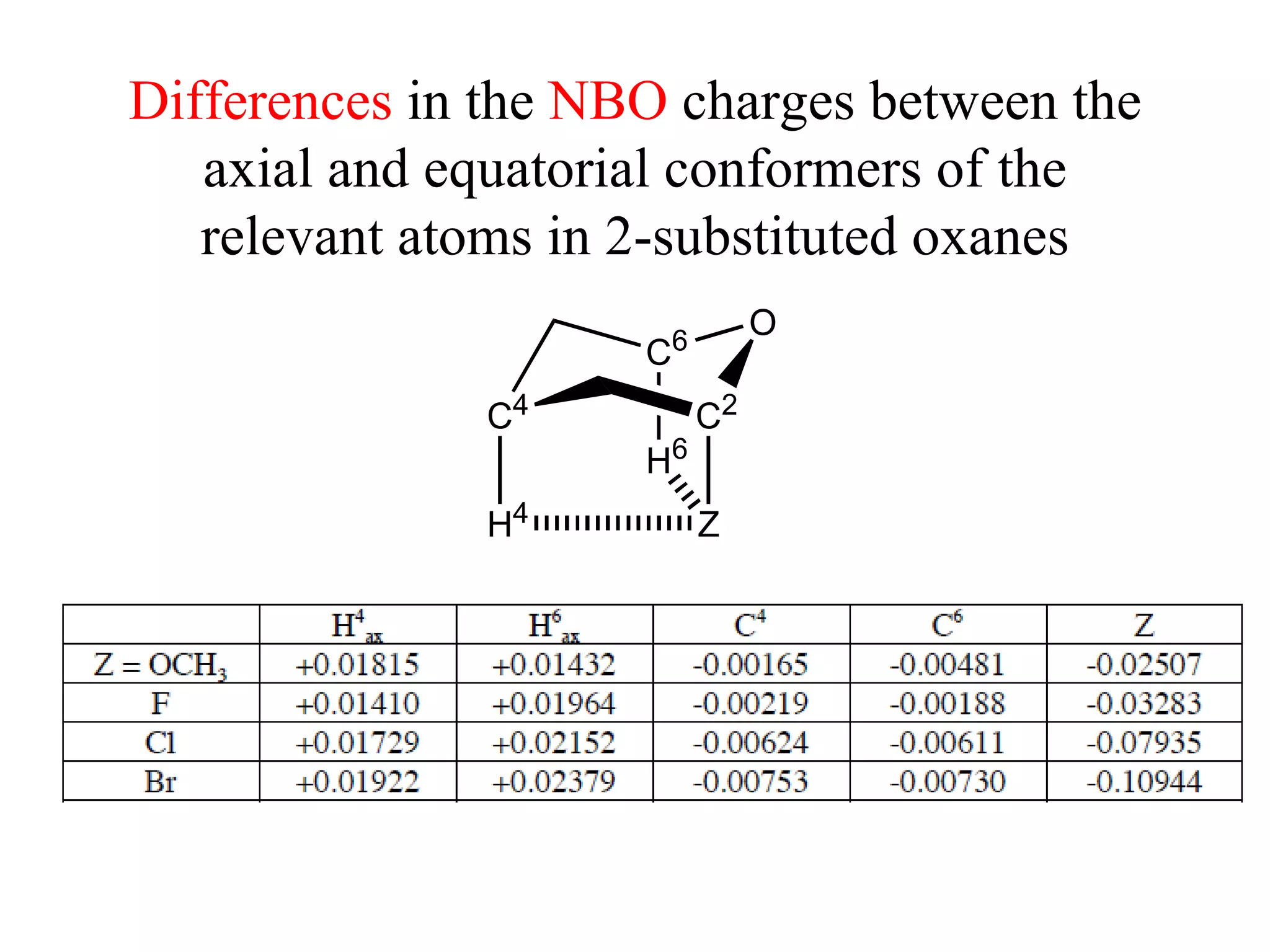
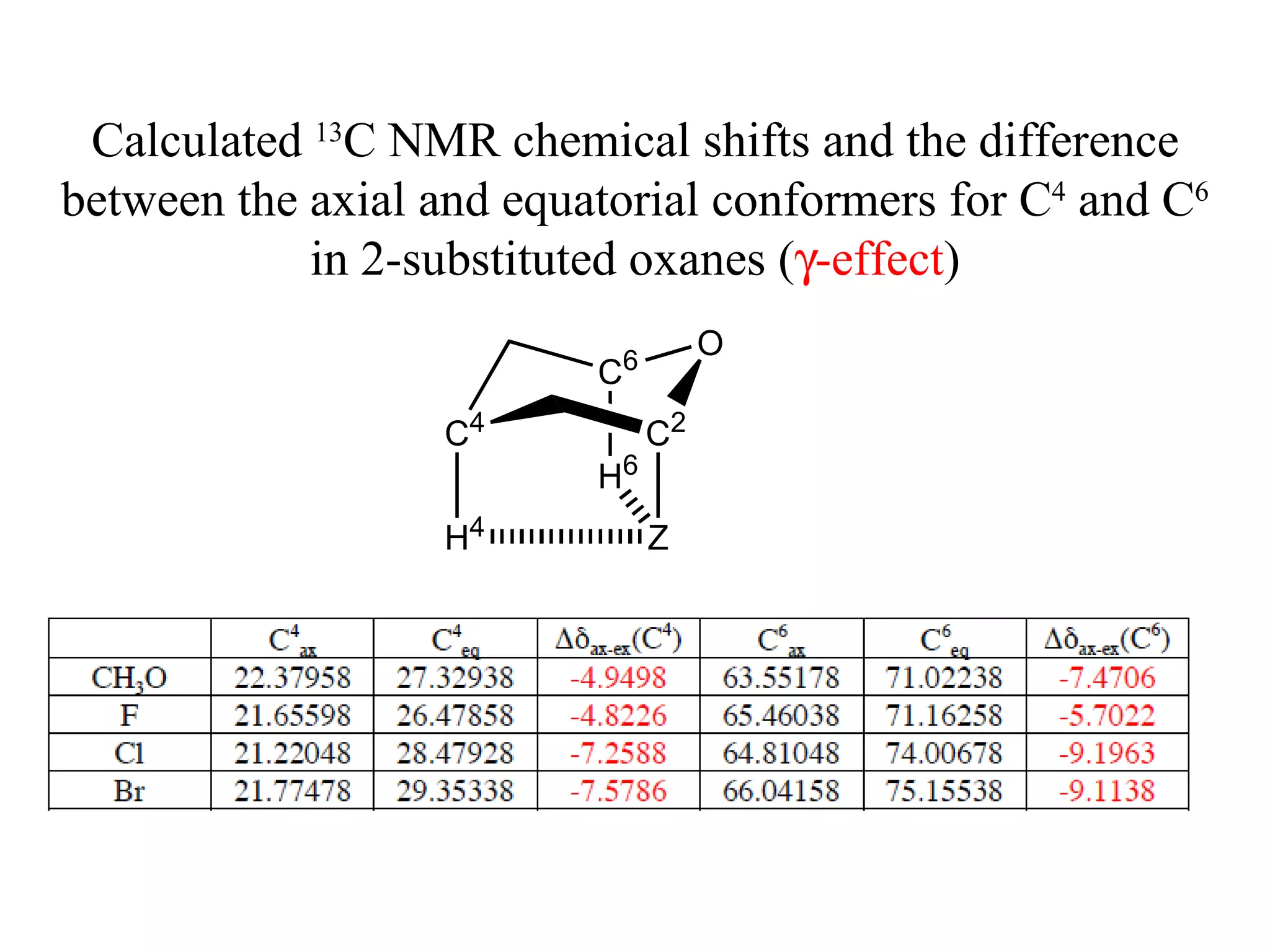
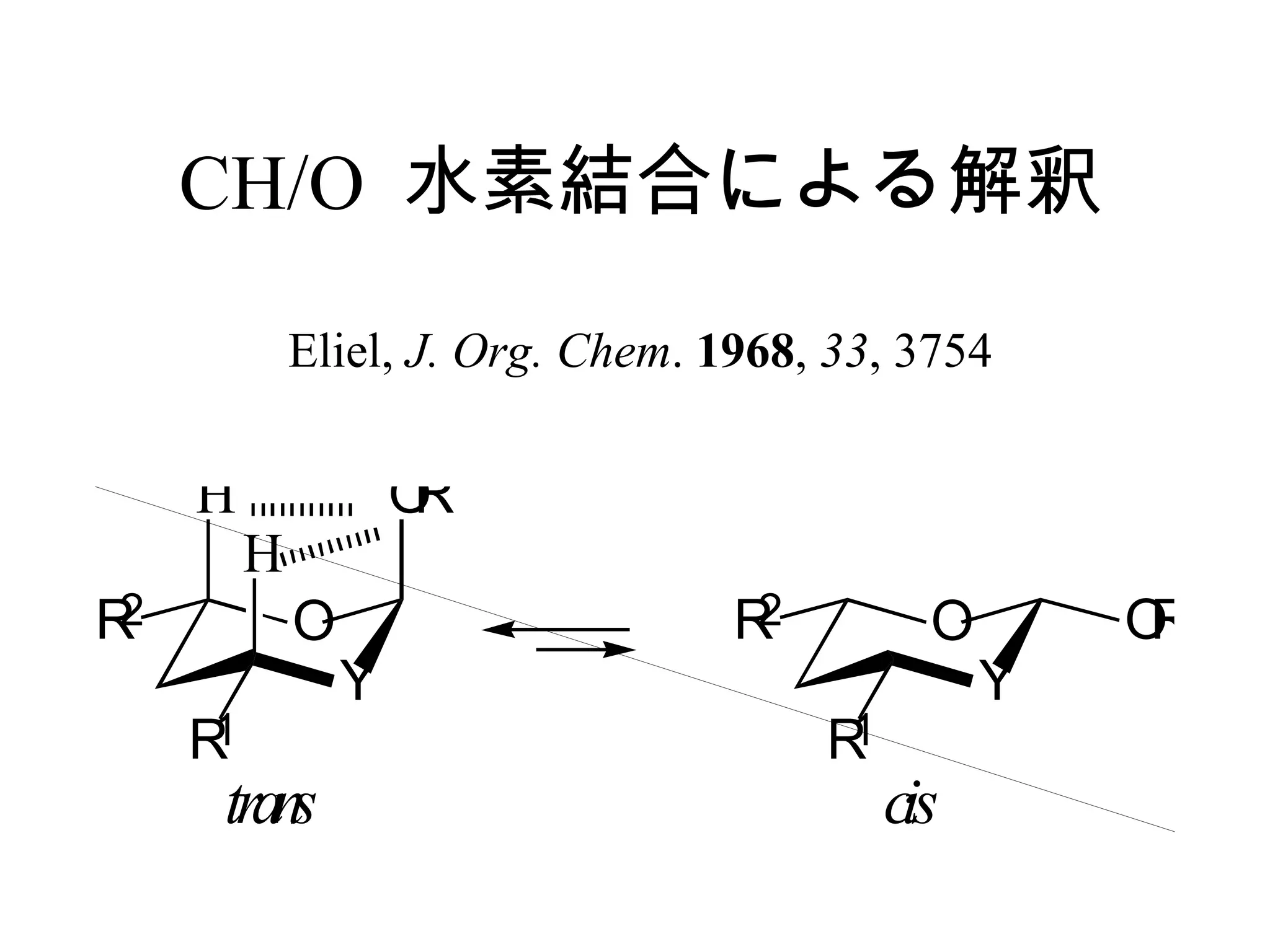
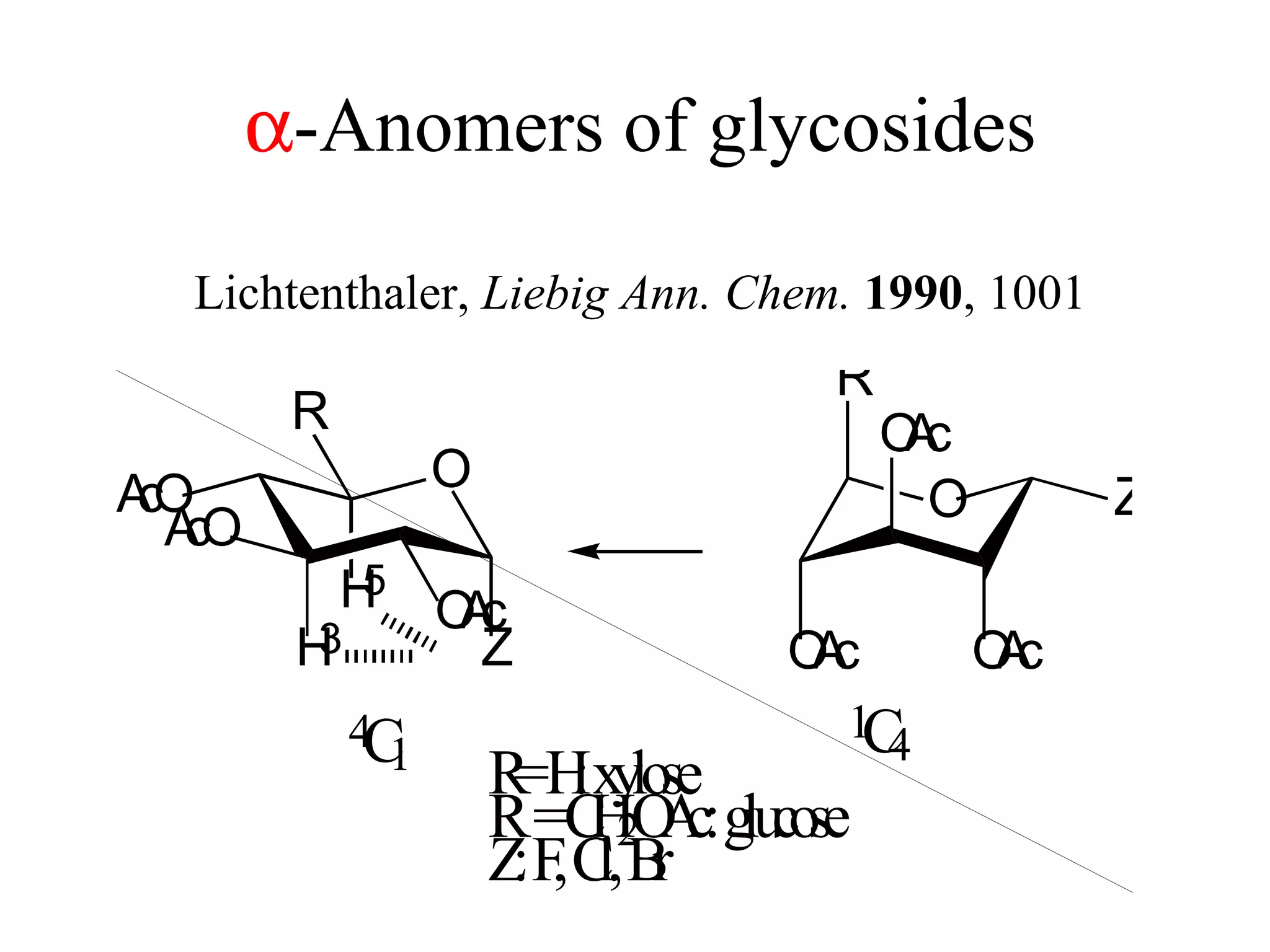
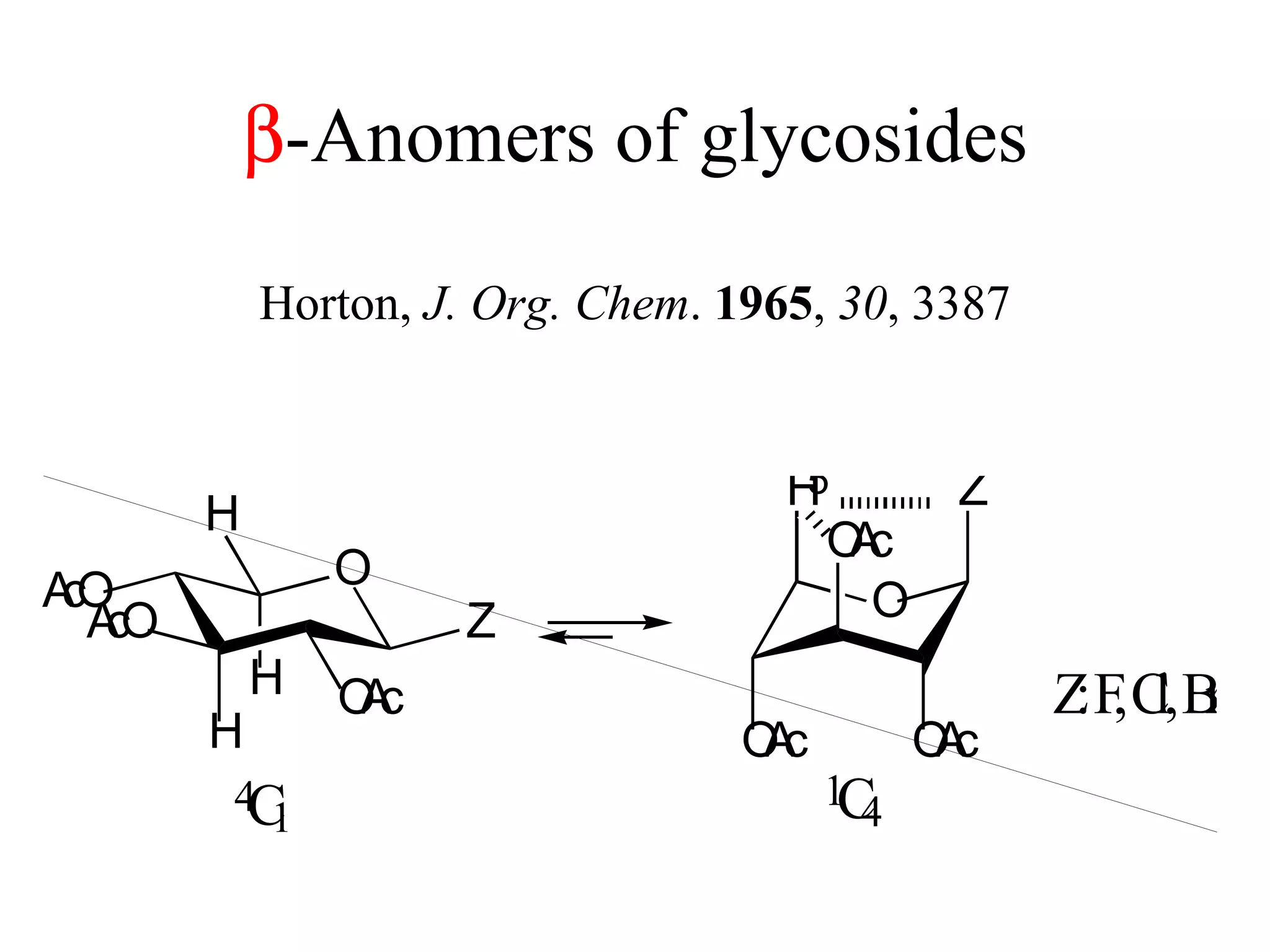
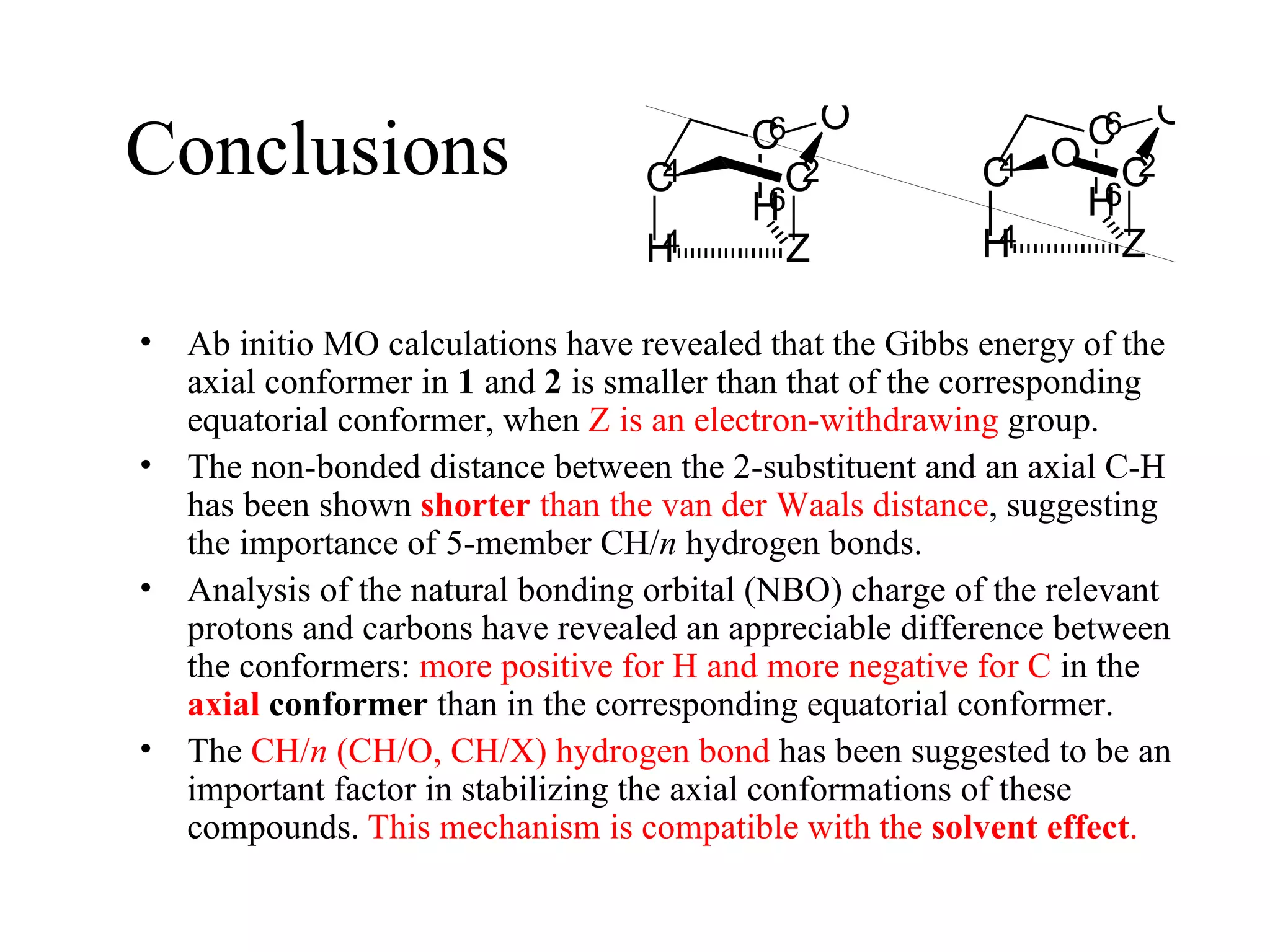
The document summarizes computational analysis of the anomeric effect in heterocyclic compounds containing CH/O or CH/X hydrogen bonds. Key findings include:
1) Ab initio calculations show the axial conformer of 1,2-substituted oxanes and 1,3-dioxanes has lower Gibbs energy than the equatorial conformer when Z is electron-withdrawing.
2) Non-bonded distances between axial hydrogens and Z are shorter than van der Waals distances, suggesting CH/n hydrogen bonds stabilize the axial conformation.
3) NBO charges reveal the axial conformer has more positive charge on axial hydrogens and more negative charge on carbons, compared to