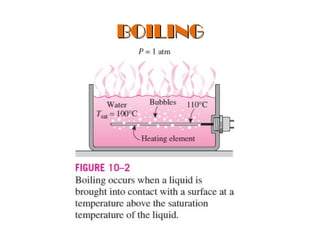
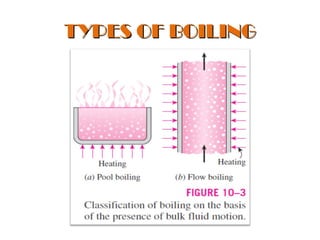
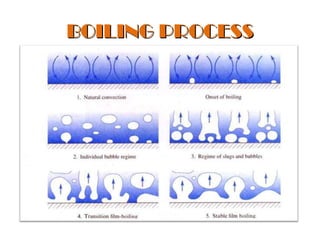
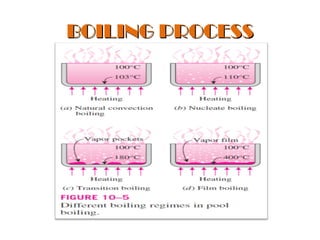
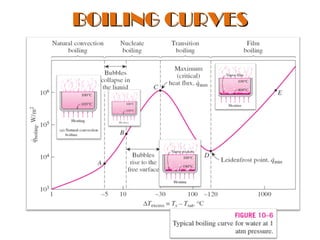
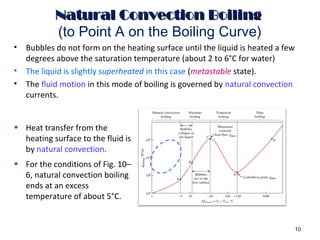
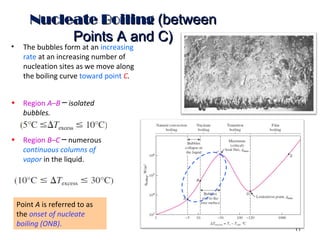
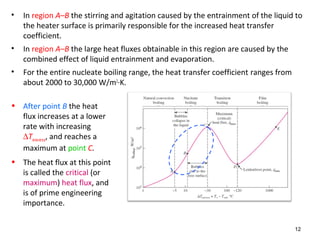
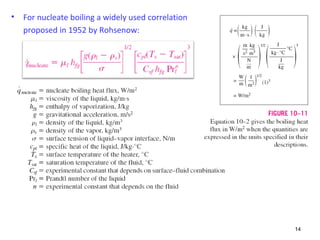
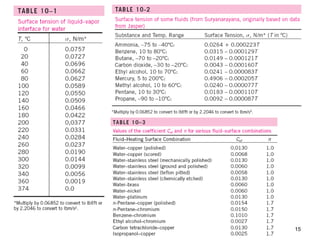
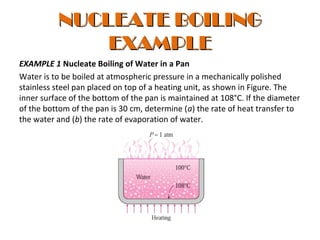
The document discusses nucleate boiling, which involves the transformation of liquid to vapor at a solid-liquid interface due to convection heat transfer. Bubbles form on the heating surface and grow before detaching. Heat transfer coefficients depend on factors like excess temperature, surface properties, and fluid properties. Nucleate boiling is important for processes like steam production, refrigeration, drying, and distillation. The document describes different boiling regimes including natural convection boiling and nucleate boiling, and provides correlations to calculate heat transfer in nucleate pool boiling. It also discusses peak heat flux and provides an example calculation.