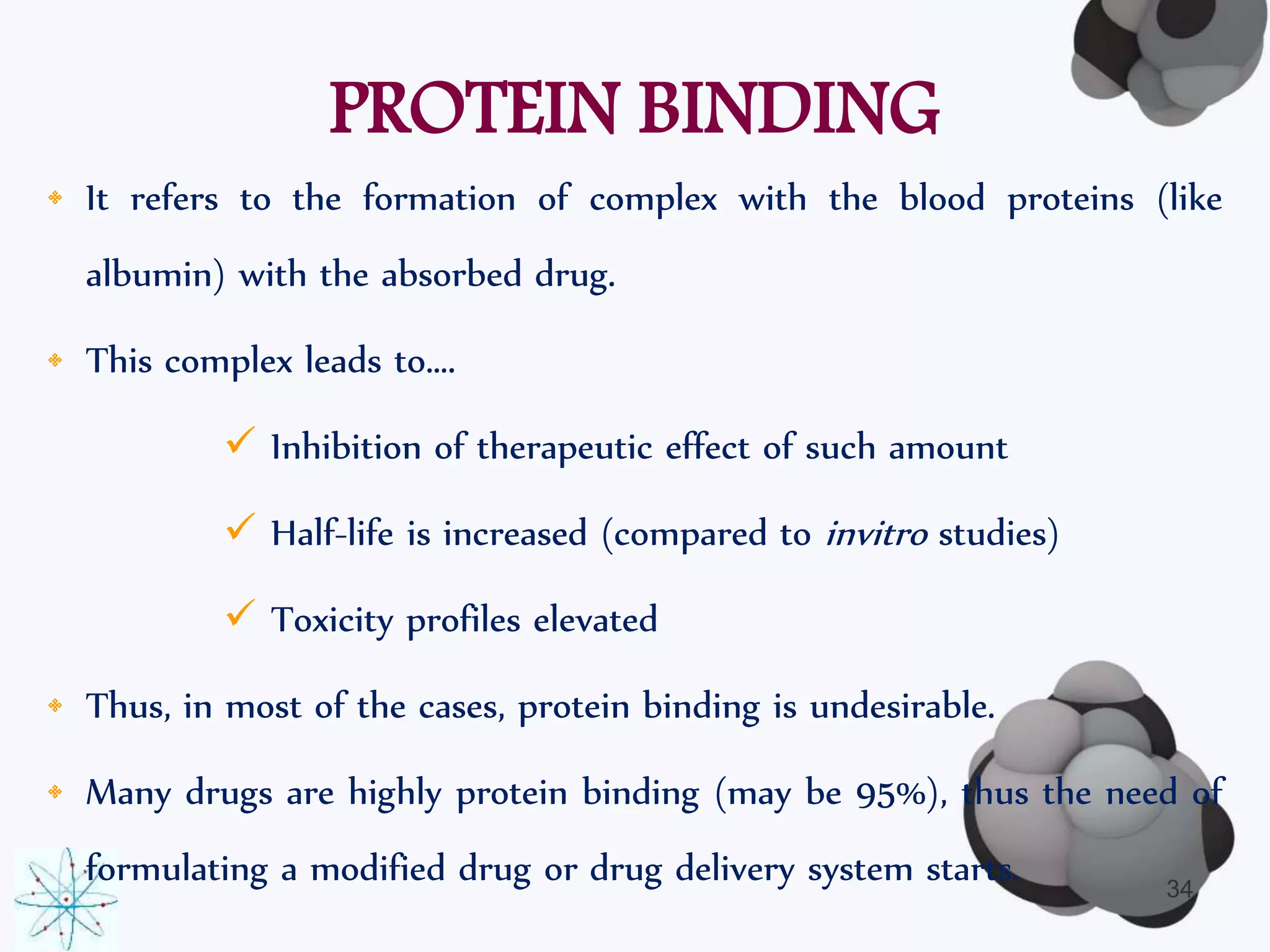
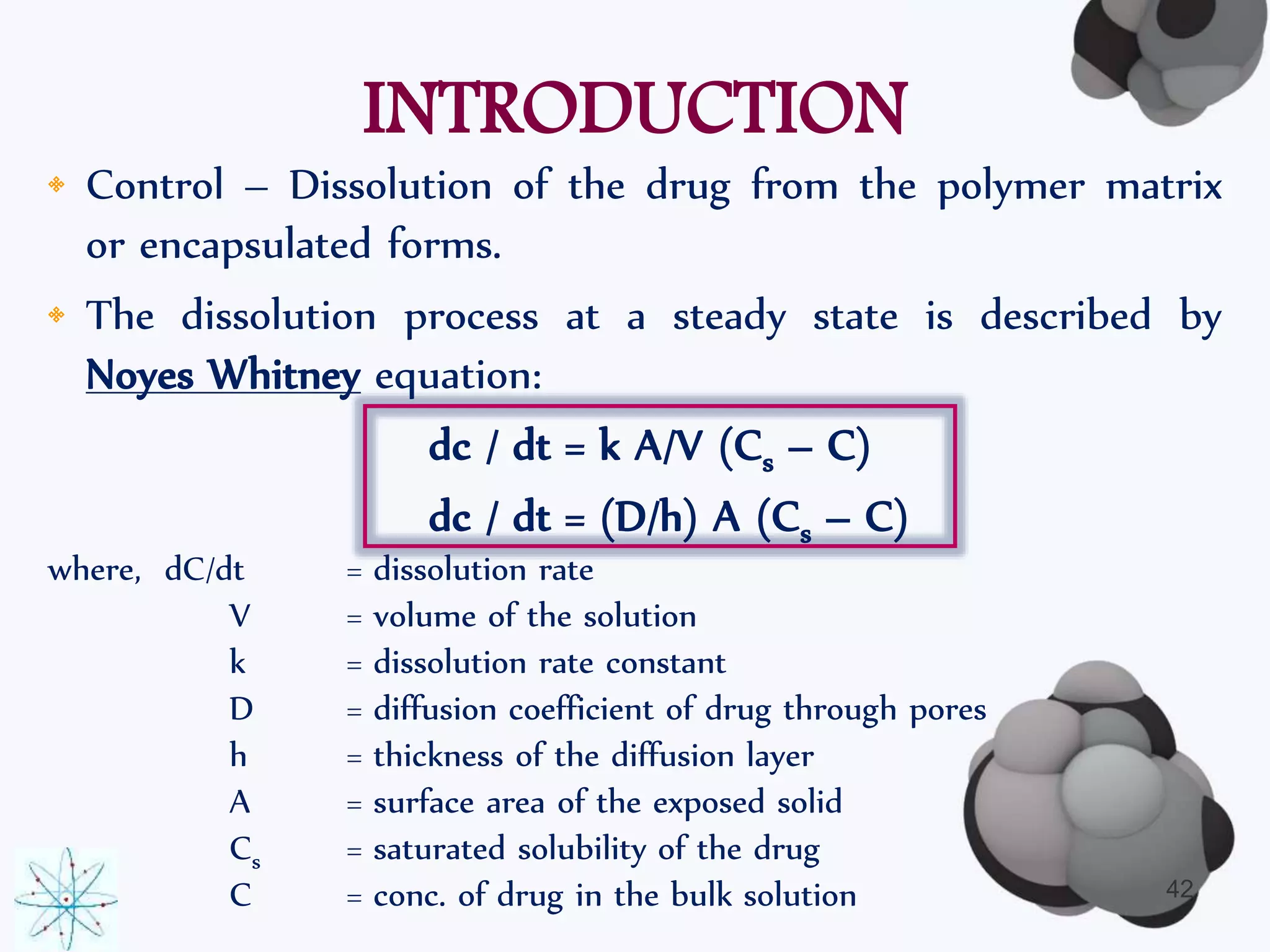
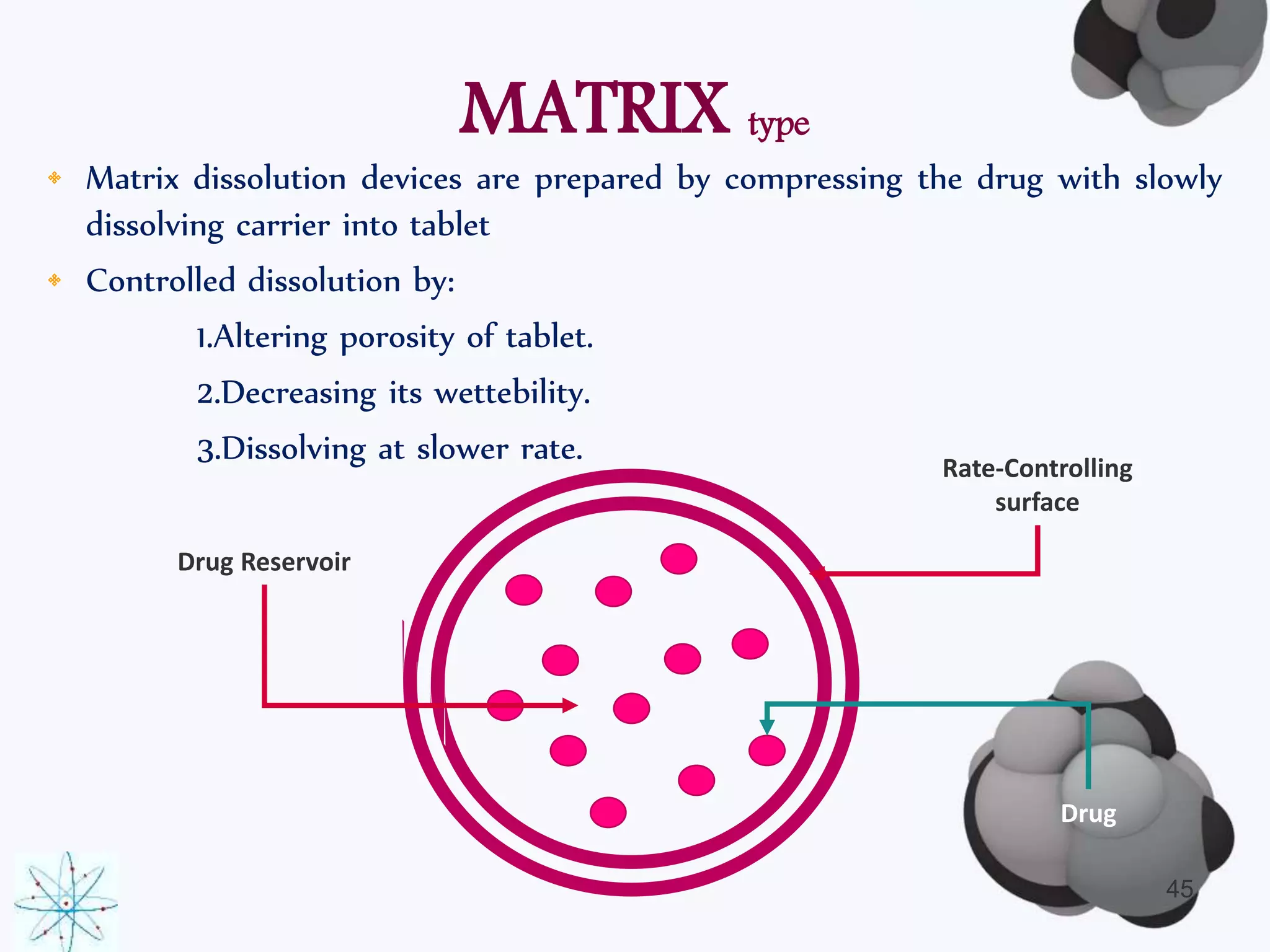
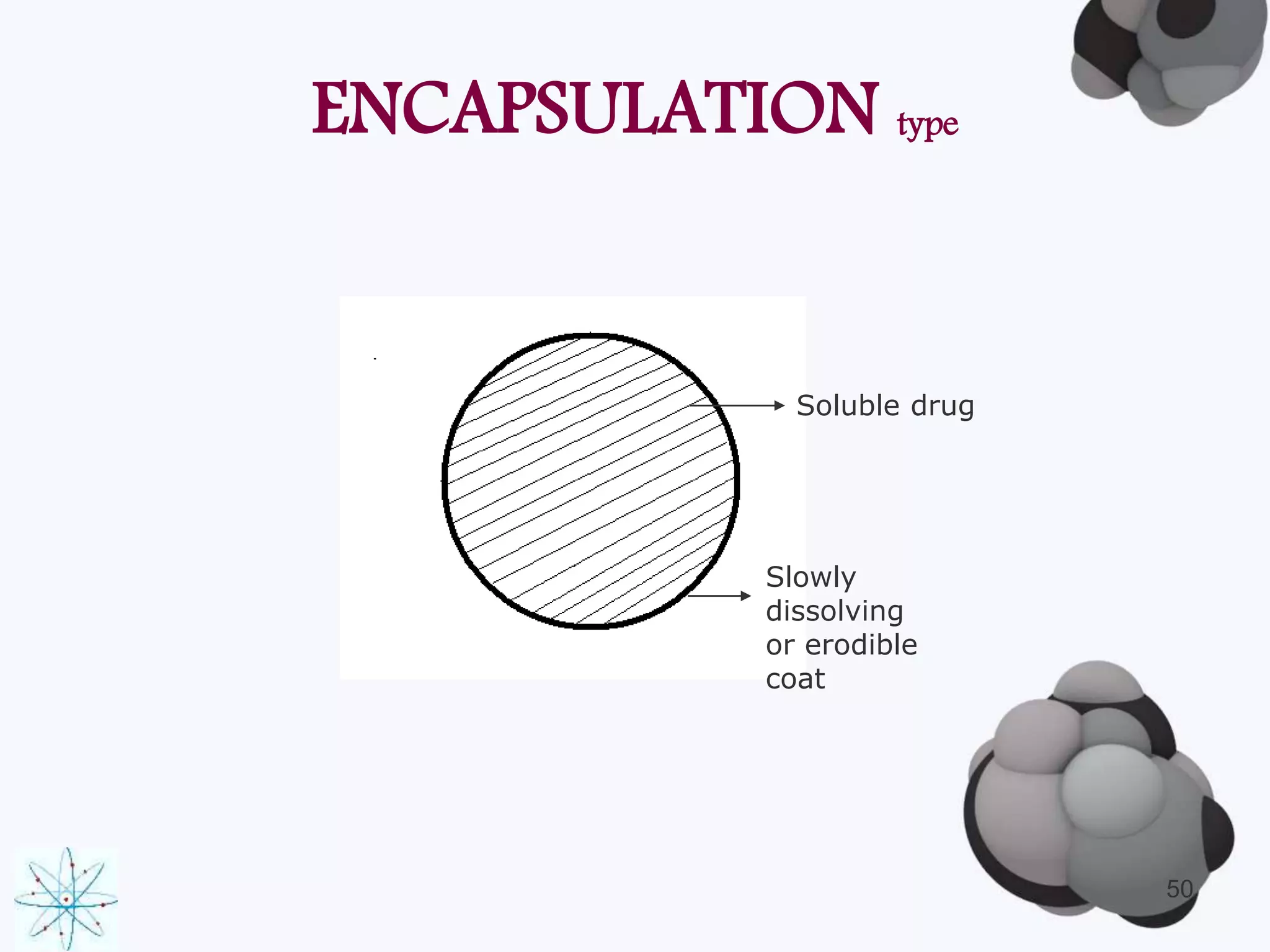
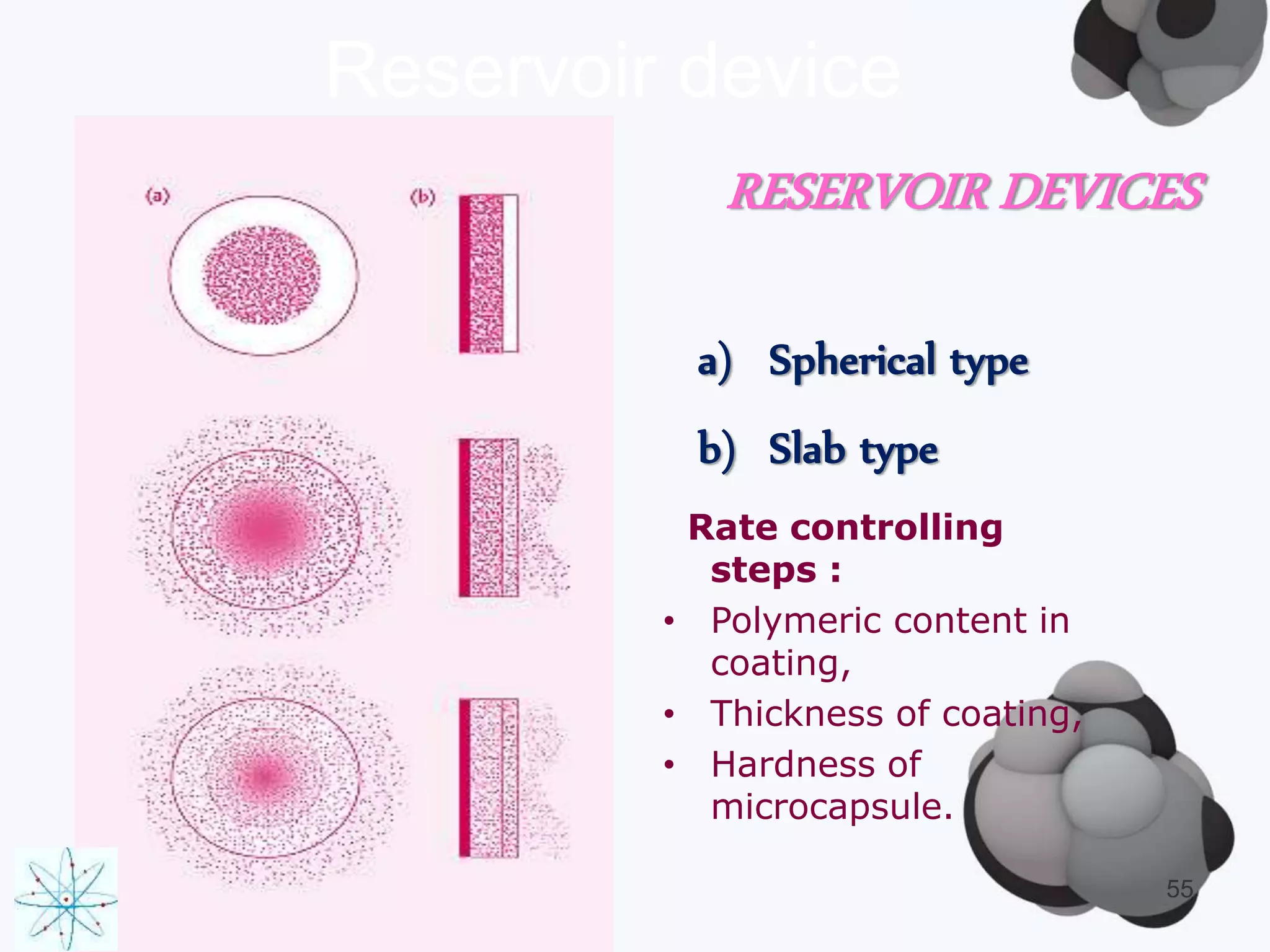
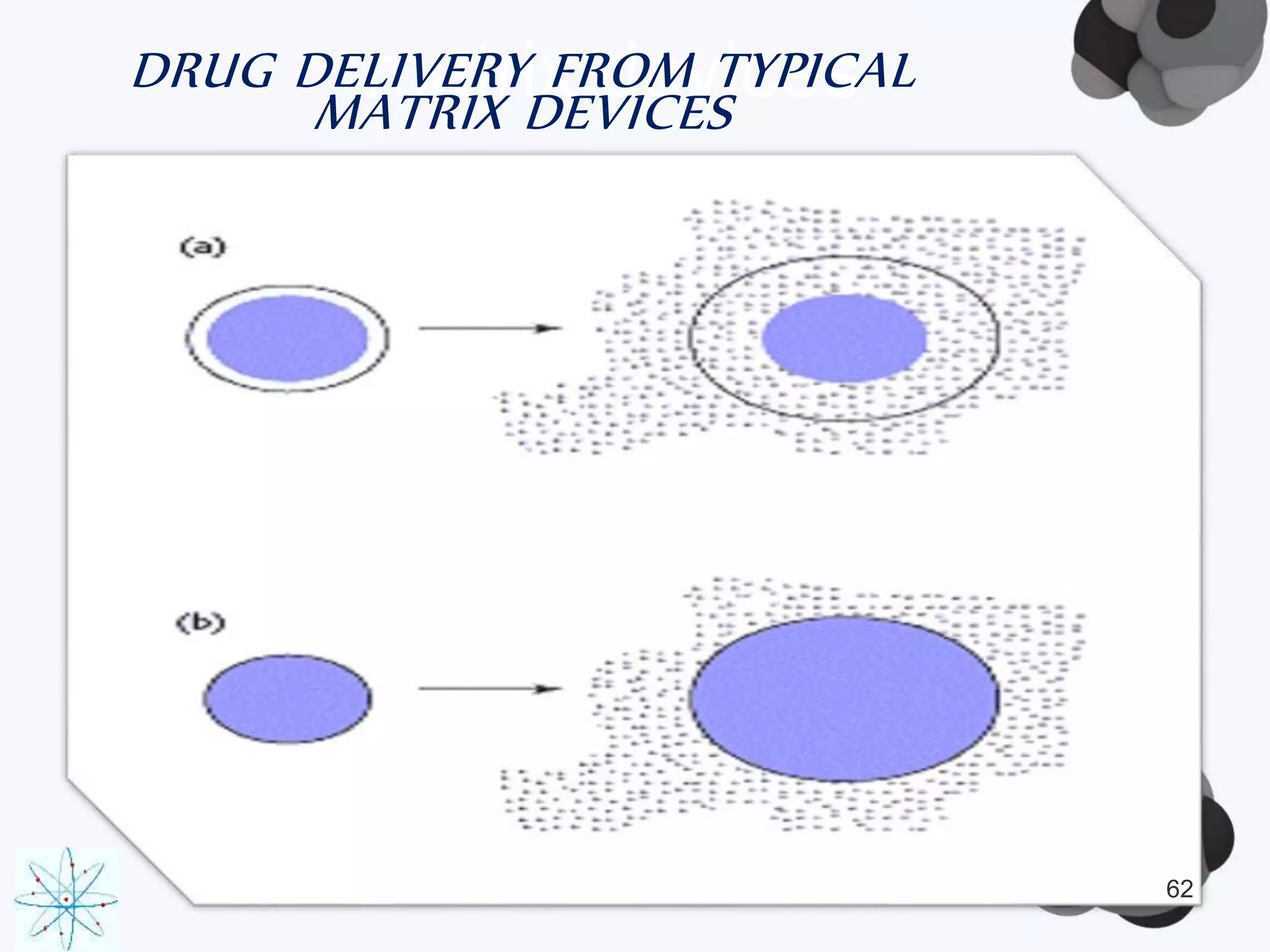
This document discusses factors affecting the design of controlled release drug delivery systems (CRDDS). It outlines several key considerations for CRDDS design including selection of the drug candidate, medical and biological rationale, and physicochemical properties. It also discusses important physicochemical factors such as solubility, partition coefficient, molecular size and diffusivity, dose size, complexation, ionization constant, drug stability, and protein binding that influence CRDDS design. Finally, it briefly describes dissolution-controlled and diffusion-controlled release approaches for developing CRDDS.













![PARTITION COEFFICIENT
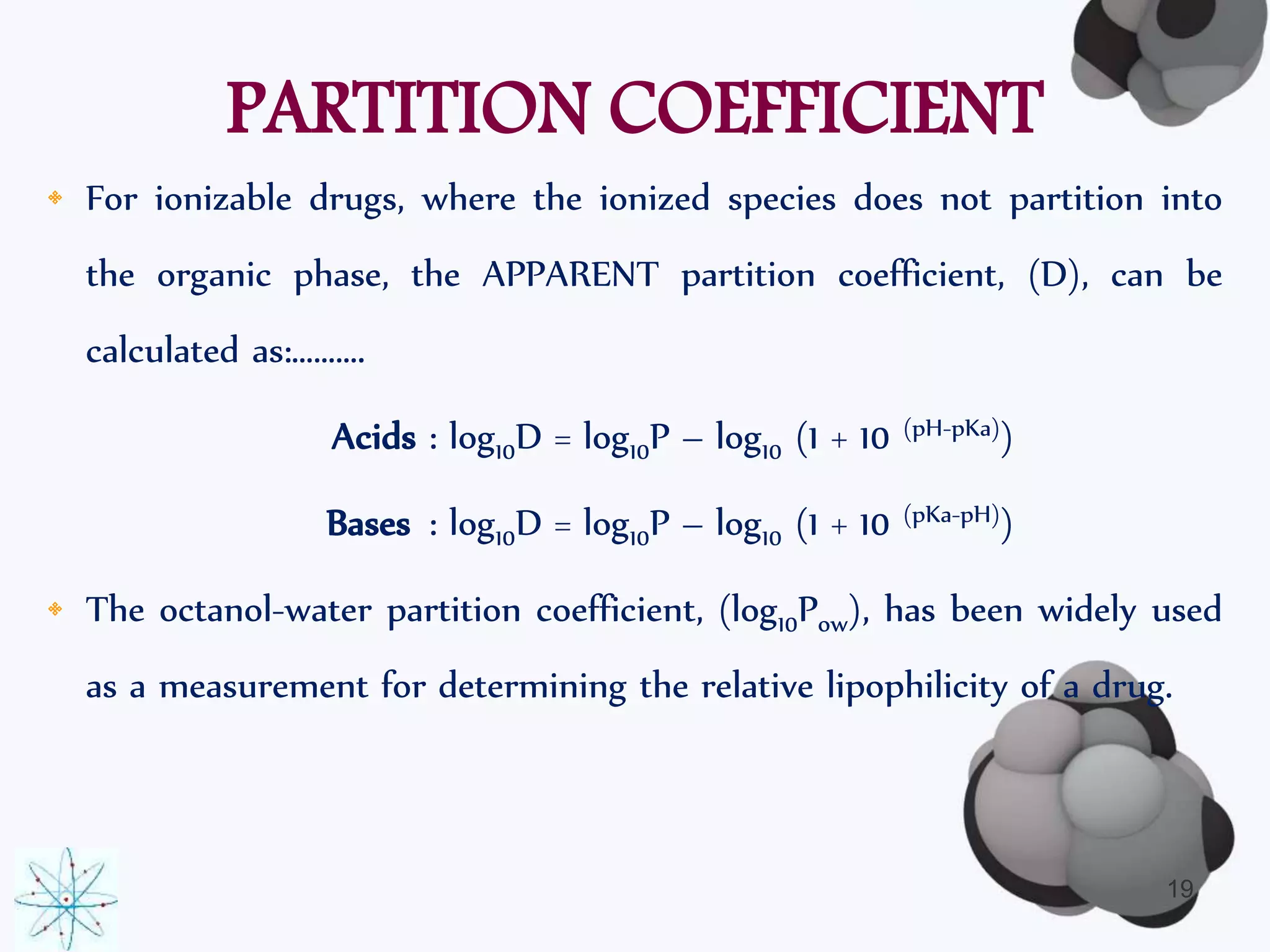
• The partition coefficient is defined as…….
“ the concentration ratio of unionized drug distributed between
two phases at equilibrium.”
• Given by the Noyes-Whitney’s Equation:
P = [퐴]표/([퐴]∞)
• The logarithm (base 10) of the partition coefficient (log10P) is often
used.
18](https://image.slidesharecdn.com/crdftypes-140919041717-phpapp02/75/Controlled-Release-Drug-Delivery-Systems-Types-Methods-and-Applications-18-2048.jpg)







![IONIZATION CONSTANT
• The Henderson-Hasselbalch eq. provides an estimate of ionized &
unionized drug conc, by function of pH…………
Acidic drugs: pKa = - log10(Ka) = pH + log10([HA]/[A-])
Basic drugs : pKa = - log10(Kb) = pH + log10([HB+]/[B-])
• Where:
Ka or Kb = ionization constant for acid/basic drugs
[HA] = conc. of unionized acid
[A-] = conc. of ionized acid
[HB+] = conc. of the unionized base
[B] = conc. of the ionized base
30](https://image.slidesharecdn.com/crdftypes-140919041717-phpapp02/75/Controlled-Release-Drug-Delivery-Systems-Types-Methods-and-Applications-30-2048.jpg)