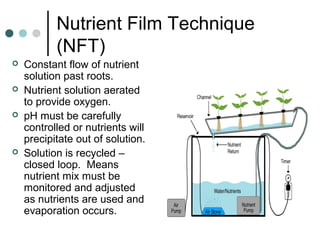
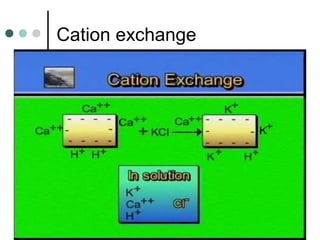
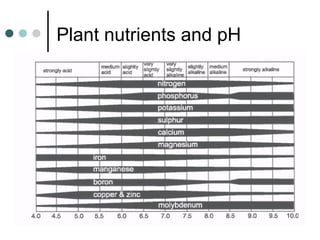
This document discusses hydroponics and plant nutrient review. It defines hydroponics as growing plants in a pH-adjusted water and nutrient solution without soil. Two common hydroponic methods are described: nutrient film technique (NFT) and substrate culture using rockwool. Environmental issues of hydroponics include water use, pollution risks, and benefits of increased land use efficiency. The review covers three macro and two micro plant nutrients and their deficiency symptoms. It also explains cation exchange capacity, soil pH, and how pH affects nutrient availability.