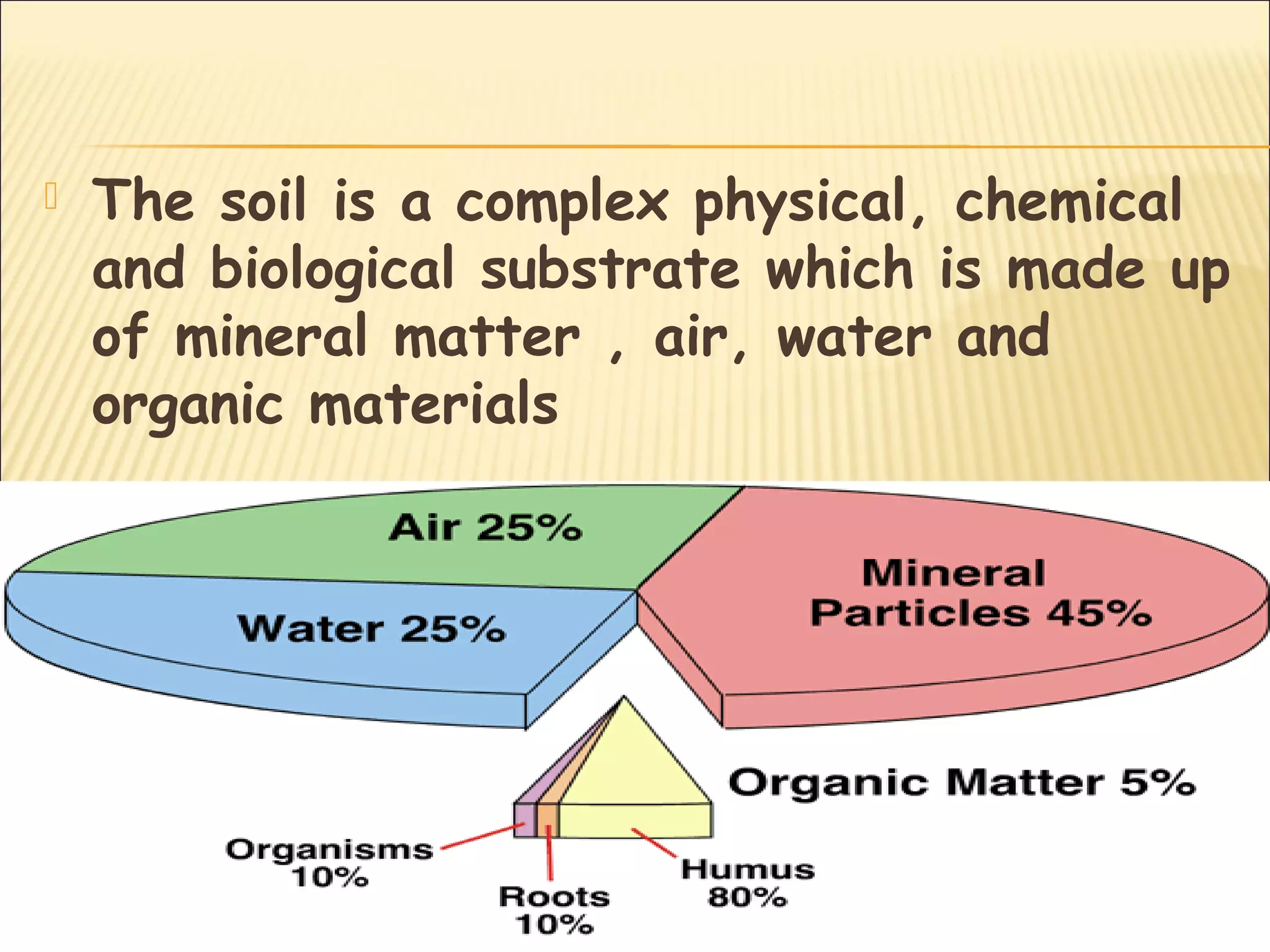
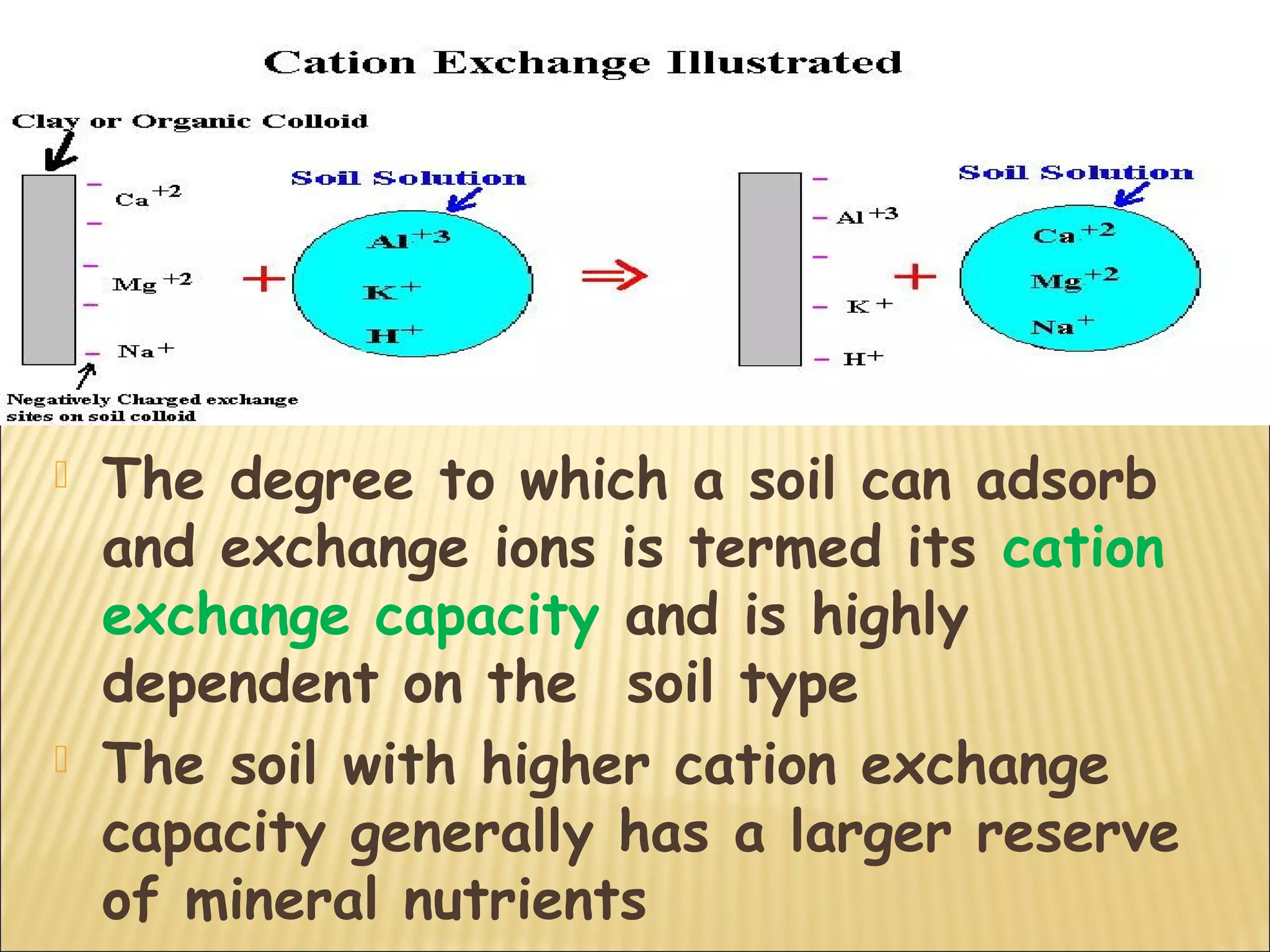
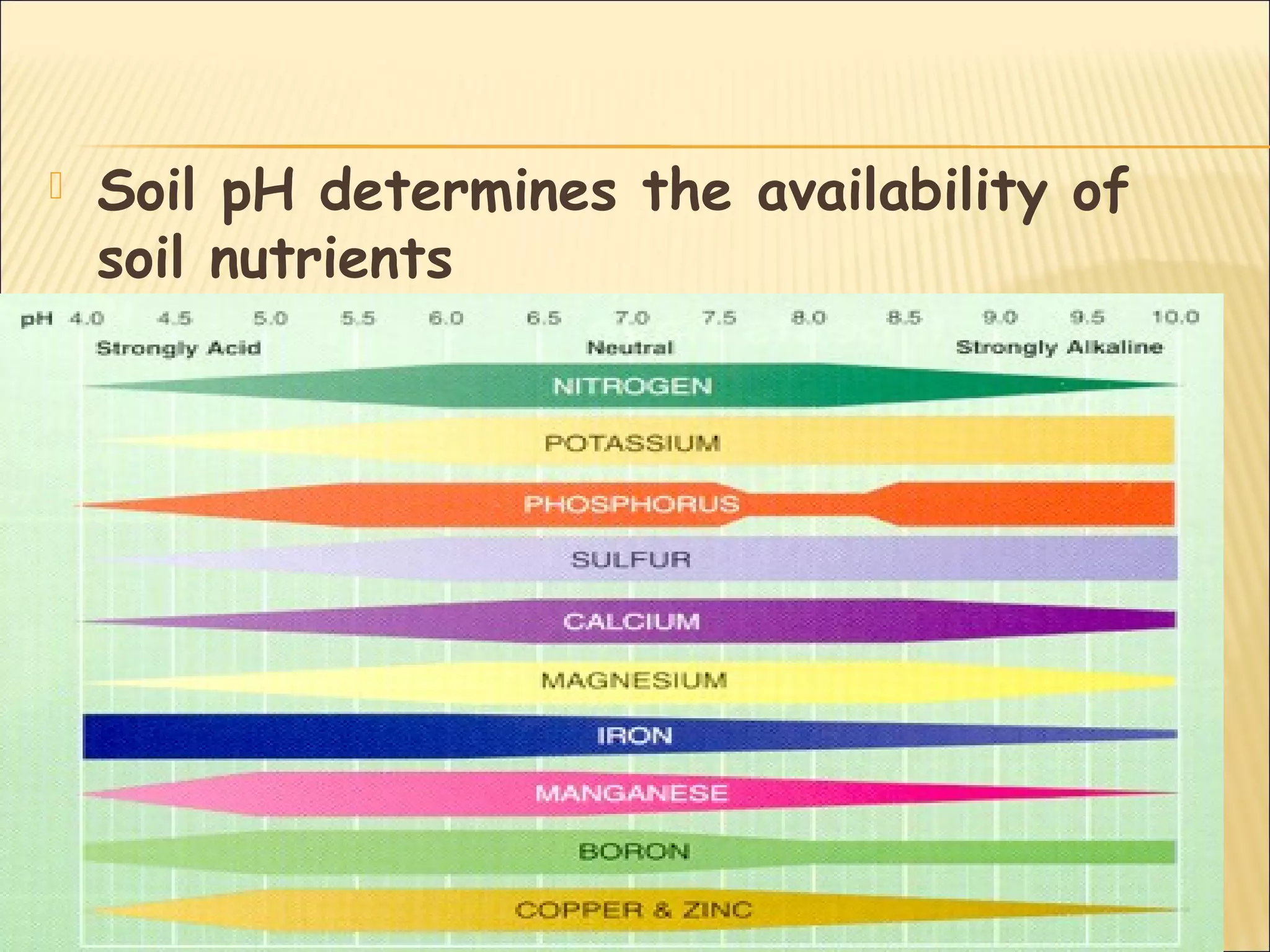
The document discusses how soil characteristics influence nutrient availability for plants. It describes the solid, liquid, and gaseous phases of soil and how they interact with mineral elements. Nutrients are obtained by plants from the soil solution, and their availability depends on factors like soil texture, particle size and charge, pH, and cation exchange capacity. Finer textured soils with more clay tend to store more nutrients due to their larger surface area for adsorption. Soil pH also affects nutrient availability and solubility.