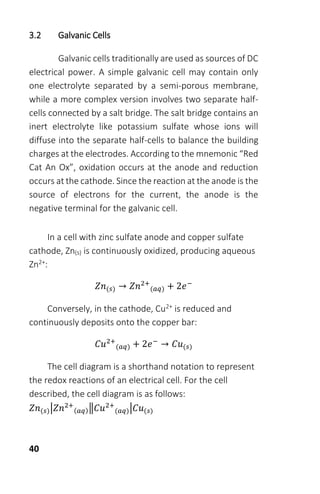
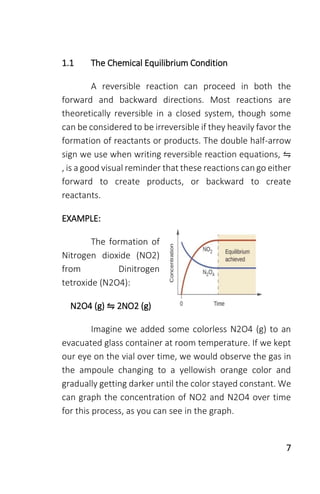
The document is a collection of solved problems in chemistry, focusing on key topics such as chemical equilibrium, acid-base equilibria, and electrochemistry. It covers critical concepts like writing equilibrium constant expressions, predicting reaction directions, and applying Le Chatelier's principle. Worksheets included in the text provide practical exercises to reinforce the understanding of these concepts.






![8
1.2 Writing the Reaction Quotient / Equilibrium
Constant Expression
The equilibrium constant expression is the ratio of
the concentrations of a reaction at equilibrium. Each
equilibrium constant expression has a constant value
known as K, the equilibrium constant. When dealing with
partial pressures, Kp is used, whereas when dealing with
concentrations (molarity), Kc is employed as the
equilibrium constant. Reactions containing pure solids and
liquids results in heterogeneous reactions in which the
concentrations of the solids and liquids are not considered
when writing out the equilibrium constant expressions.
𝐾𝑐 =
[C]c
[D]d
[A]a
[B]b 𝐾𝑝 =
𝑃 𝐶
𝑐
𝑃 𝐷
𝑑
𝑃 𝐴
𝑎 𝑃 𝐵
𝑏
There are two different types of equilibria (homogeneous
and heterogeneous) separately, because the equilibrium
constants are defined differently.
• A homogeneous equilibrium has everything
present in the same phase.
• A heterogeneous equilibrium has things present in
more than one phase.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-8-320.jpg)






![15
SOLUTION:
1. Given: 2NO + 2H2 ⇌ N2 + 2H2O
Where: NO= 0.789M, H2= 0.016M, N2= 0.214M, H2O=
0.14M
Solution: 𝐾𝑐 =
[C]c[D]d
[A]a[B]b
𝐾𝑐 =
[0.214][0.14]
[0.789]2[0.016]2
𝐾𝑐 = 187.996
Answer : The equilibrium Constant Kc = 187.996
2. Given: C2H5OH(l) + O2(g) ⇌ 2CO2(g) + H2O(g)
Where: C2H5OH= 0.189M, O2= 0.19M, CO2= 0.712M,
H2O= 0.18M
Solution: 𝐾𝑐 =
[C]c[D]d
[A]a[B]b
𝐾𝑐 =
[0.712]2
[0.18]
[0.19]
𝐾𝑐 = 0.48
Answer : The equilibrium Constant Kc = 0.48](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-15-320.jpg)
![16
3. Given: C2H4 (g) + 2O2 (g) ⇌ CO2(g) + 2H2O(g)
Where: C2H4= 0.28M, O2= .0085M, CO2= 2.12M, H2O=
0.12M
Solution: 𝐾𝑐 =
[C]c[D]d
[A]a[B]b
𝐾𝑐 =
[2.12][0.12]2
[0.28][0.0085]2
𝐾𝑐 = 1509.046
Answer : The equilibrium Constant Kc = 1509.046
4. 2H2O2 ⇌ 2 H2O +O2
Given: V= 750 m, T= 618 K, grams H2O2
Find: Pressure of the O2 produced in atmospheres
a. First, we need to determine the moles of O2
produced.
(9g H2O2 ) × (
1 𝑚𝑜𝑙 H2O2
34𝑔 H2O2
) × (
1 𝑚𝑜𝑙 O2
2 𝑚𝑜𝑙 H2O2
)
= 0.13 𝑚𝑜𝑙 O2
b. With the moles of Oxygen determined, we can now use
the Ideal Gas Law to determine the pressure.
PV = nRT](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-16-320.jpg)


![19
Cl2 =
0.00618
1.5
= 4.12 x 10−3
M
HCl =
0.61816
1.5
= 0.41 M
Solving Qc
𝑄 𝑐 =
[0.41]2
[0.166][4.12 × 10−3]
𝑄 𝑐 = 245.79
Because Qc is smaller than Kc, the system is not at
equilibrium. The net result will be an increase in the
concentration of HCl and a decrease in the concentrations
of H2 and Cl2. The net reaction will proceed from left to right
until equilibrium is reached.
9. H2(g)+I2(g) ⇌ 2HI(g)
Given: 0.718 mol H2 0.82 mol I2 Kc=
2.35 V= 4.2L
0.0052 mol HI
The initial concentrations of the reacting species
H2 =
0.718
4.2
= 0.17 M HI =
0.0052
4.2
= 1.24 x 10−3
M
I2 =
0.82
4.2
= 0.2 M](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-19-320.jpg)
![20
Solving Qc:
𝑄 𝑐 =
[1.24 x 10−3
]2
[0.17][0.2]
𝑄 𝑐 = 0.04
Because Qc is smaller than Kc, the system is not at
equilibrium. The net result will be an increase in the
concentration of HI and a decrease in the concentrations of
H2 and I2. The net reaction will proceed from left to right
until equilibrium is reached.
10. 2NO (g) + 2H2 (g) ⇌ N2 (g) + 2H2O (g)
Given: NO= 0.789M N2= 0.214M Kc= 10.18
H2= 0.016M H2O= 0.14M
Solve Qc:
𝑄𝑐 =
[0.214𝑀][0.98𝑀]2
[0.789𝑀]2[0.16]2
𝑄𝑐 = 12.9
Because Qc is bigger than Kc, the system is not at
equilibrium. The net result will be an increase in the
concentrations of NO and H2 and a decrease in the
concentrations of N2 and H2O. The net reaction will proceed
from left to right until equilibrium is reached.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-20-320.jpg)

![22
Key Points:
• Acid is a substance that contains hydrogen and
dissociates in water to yield a hydronium ion.
• A base is a substance that contains the hydroxyl group
and dissociates in water to yield : OH-
• Acid/Base theory and equilibria focus on the
relationship of these two ions and how they each affect
the equilibria of a whole class of compounds known as
acids and bases.
• Weak base is a chemical base that does not ionize fully
in an aqueous solution.
• The acid dissociation constant (Ka) quantifies the extent
of dissociation of a weak acid. The larger the value of
Ka, the stronger the acid, and vice versa.
• The base dissociation constant (or base ionization
constant) Kb quantifies the extent of ionization of a
weak base. The larger the value of Kb , the stronger the
base, and vice versa.
The General Weak Acid-Base Equilibria Formulas:
For a generic monoprotic weak acid HA with
conjugate base A- , the equilibrium constant has the
form: 𝐊 𝐚 =
[𝐇 𝟐 𝐎+][𝐀−]
[𝐇𝐀]
For a generic weak base B with conjugate acid BH+,
the equilibrium constant has the form: 𝐊 𝐛 =
[𝐁𝐇+][𝐎𝐇−]
[𝐁]](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-22-320.jpg)




![27
2.3 pH- A Measure of Acidity
pH is a measure of hydrogen ion concentration; a
measure of the acidity or alkalinity of a solution. The pH
scale usually ranges from 0 to 14. Aqueous solutions at 25°C
with a pH less than seven are acidic, while those with a pH
greater than seven are basic or alkaline. A pH level of is 7.0
at 25°C is defined as 'neutral'. Very strong acids may have a
negative pH, while very strong bases may have a pH greater
than 14.
pH Equation: To calculate the pH of an aqueous
solution you need to know the concentration of the
hydronium ion in moles per liter (molarity). The pH is then
calculated using the expression:
pH = -log[H+]
where log is the base-10 logarithm and [H+] stands for the
hydrogen ion concentration in units of moles per liter
solution.
OH Equation: To calculate the pOH of a solution
you need to know the concentration of the hydroxide ion in
moles per liter (molarity). The pOH is then calculated using
the expression:
pOH = - log [OH-]
Where log is the base-10 logarithm and [H+] stands for the
hydrogen ion concentration in units of moles per liter
solution.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-27-320.jpg)

![29
2.5 Weak Acids/Weak Bases
Weak acids and bases are only partially ionized in
their solutions, whereas strong acids and bases are
completely ionized when dissolve in water. Some common
weak acids and bases are given here. Furthermore, weak
acids and bases are very common, and we encounter them
often both in the academic problems and in everyday life.
The ionization of weak acids and bases is a chemical
equilibrium phenomenon. The equilibrium principles are
essential for the understanding of equilibria of weak acids
and weak bases.
Because the acid is weak, an equilibrium expression
can be written. An acid ionization constant (Ka) is the
equilibrium constant for the ionization of an acid.
𝐾𝑎 =
[ 𝐻+][𝐴−]
[𝐻𝐴]
The numerical value of K_b is a reflection of the
strength of the base. Weak bases with relatively higher K_b
values are stronger than bases with relatively lower K_b
values.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-29-320.jpg)
![30
2.6 Relationship between the Ionization Constants of
Acids and their Conjugate Bases
As with acids, bases can either be strong or weak,
depending on their extent of ionization.A weak base is a
base that ionizes only slightly in an aqueous solution.When
a weak base such as ammonia is dissolved in water, it
accepts an H + ion from water, forming the hydroxide ion
and the conjugate acid of the base, the ammonium ion.
𝑁𝐻3 + 𝐻2 𝑂 ⇆ 𝑁𝐻4
+
+ 𝑂𝐻−
An equilibrium expression can be written for the
reactions of weak bases with water. Because the
concentration of water is extremely large and virtually
constant, the water is not included in the expression. A base
ionization constant (𝐾𝑏) is the equilibrium constant for the
ionization of a base. For ammonia the expression is:
𝐾𝑏 =
[𝑁𝐻4
+
][𝑂𝐻−
]
[𝑁𝐻3]
The numerical value of 𝐾𝑏 is a reflection of the
strength of the base. Weak bases with relatively higher 𝐾𝑏
values are stronger than bases with relatively lower 𝐾𝑏
values.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-30-320.jpg)
![31
2.7 The Common Ion Effect
The common ion effect states that in a chemical solution, if
the concentration of any one of the ions is increased, then,
according to Le Chatelier's principle, some of the ions in
excess should be removed from solution, by combining
with the oppositely charged ions. Some of the salt will be
precipitated until the ion product is equal to the solubility
product. In short, the common ion effect is the suppression
of the degree of dissociation of a weak electrolyte
containing a common ion.
The solubility products Ksp's are equilibrium constants in
hetergeneous equilibria (i.e., between two different
phases). If several salts are present in a system, they all
ionize in the solution. If the salts contain a common cation
or anion, these salts contribute to the concentration of the
common ion. Contributions from all salts must be included
in the calculation of concentration of the common ion. For
example, a solution containing sodium chloride and
potassium chloride will have the following relationship:
[Na+]+[K+]=[Cl−]
When NaCl and KCl are dissolved in the same
solution, the Cl− ions are common to both salts. In a system
containing NaCl and KCl, the Cl− ions are common ions.](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-31-320.jpg)

![33
2.9 Solubility Equilibria
The solubility product is the equilibrium constant
representing the maximum amount of solid that can be
dissolved in aqueous solution. The general form of the
solubility product constant (Ksp) for the equation: aA (s) ⇌
bB (aq) + cC (aq) is Ksp = [B]b[C]c
The solubility product constant (Ksp) is the equilibrium
constant for a solid that dissolves in an aqueous solution.
All of the rules for determining equilibrium constants
continue to apply. An equilibrium constant is the ratio of
the concentration of the products of a reaction divided by
the concentration of the reactants once the reaction has
reached equilibrium. Consider this reaction:
𝐴𝑔𝐶𝑙(𝑠) → 𝐴𝑔+
(𝑎𝑞) + 𝐶𝑙−
(𝑎𝑞)
The equilibrium expression for the reaction is:
𝐾 =
[𝐴𝑔+][𝐶𝑙−
]
[𝐴𝑔𝐶𝑙]
Because the AgCl is a solid, its concentration before
and after the reaction is the same. The equilibrium
equation can therefore be rearranged as: 𝑲 𝒔𝒑 =
[𝑨𝒈+][𝑪𝒍−
]
For substances in which the ions are not in a 1:1 ratio,
the stoichiometric coefficients of the reaction become the
exponents for the ions in the solubility-product expression.
The general formula for a reaction 𝑎𝐴(𝑠) ⇌ 𝑏𝐵(𝑎𝑞) + 𝑐𝐶(𝑎𝑞)
is: 𝑲 𝒔𝒑 = [𝑩] 𝒃
[𝑪] 𝒄](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-33-320.jpg)
![34
WORKSHEET #2
1. Find the pH of a 0.0125 M HCl solution. The HCl is a
strong acid and is 100% ionized in water. The
hydronium ion concentration is 0.0025 M.
2. Calculate the pH for a specific [H+]. Calculate pH given
[H+] = 1.8 x 10-5 M
3. Calculate [H+] from a known pH. Find [H+] if pH = 7.8
4. What is the pOH of a solution that has a hydroxide ion
concentration of 6.18 x 10-5 M?
5. What is the hydroxide ion concentration in a solution
that has a pOH of 6.90?
6. Calculate the pH for a specific [H+]. Calculate pH given
[H+] = 6 M
7. Calculate the pH for a specific [H+]. Calculate pH given
[H+] = 2.89 M
8. Given the H+ concentration to be 6.18×106, what is the
pH? Identify if it is acidic, basic or neutral.
9. Express the solubility equilibrium constant of the
following dissociation equation: NaCl⇌ Na+ + Cl-
10. Express the solubility equilibrium constant of the
following dissociation equation: Fe2(SO4)3 ⇌ 2Fe3+ +
3SO4
2-](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-34-320.jpg)
![35
SOLUTION:
1. Given: 0.0025M HCl
Solution: pH = - log (0.0125)
= - ( - 2.90) = 2.90
Answer : pH= 2.90
2. Given: [H+] = 1.8 x 10-5 M
Solution: pH = - log (1.8 x 10-5)
= - ( - 4.74) = 4.74
Answer : pH= 4.74
3. Given: pH = 7.8
Solution: [H+] = 10-pH
[H+] = 10-7.8
[H+] = 1.58 x 10-8
Answer : [H+] = 1.58 x 10-8
4. Given: 6.18 x 10-5 M
pOH = - log [6.18 x 10-5]
= - ( - 4.22) = 4.21
Answer : pOH= 4.21
5. Given: pOH of 6.90
Solution: [OH-] = 10-pOH
[OH-] = 10-6.90
[OH-] = 1.26 x 10-7
Answer : [OH-] = 1.26 x 10-7
6. Given: [H+] = 6 M
Solution: pH = - log (6)
= - (-0.78) = 0.78](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-35-320.jpg)
![36
Answer : pH= 0.78
7. Given: [H+] = 2.89 M
Solution: pH = - log (2.89)
= - ( - 0.46) = 0.46
Answer : pH= 0.46
8. Given: [H+] = 6.18×106M
Solution: pH = - log (6.18×106)
= - ( - 6.79) = 6.79
Answer : pH= 6.79 and therefore ACIDIC
9. Express the solubility equilibrium constant of the
following dissociation equation. NaCl⇌ Na+ + Cl-
Answer : 𝐾𝑠𝑝 = [𝑁𝑎+][𝐶𝑙−
]2
10. Express the solubility equilibrium constant of the
following dissociation equation. Fe2(SO4)3 ⇌ 2Fe3+
+ 3SO4
2-
Answer : 𝐾𝑠𝑝 = [𝐹𝑒3+]2
[𝑆𝑂42
2−
]
3](https://image.slidesharecdn.com/generalchemistryii-180414161432/85/Workbook-for-General-Chemistry-II-36-320.jpg)