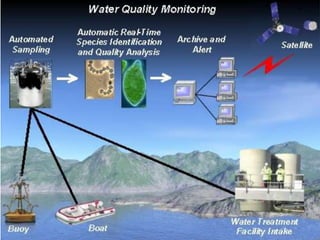
Water quality monitoring is a fundamental tool for managing freshwater resources. It refers to measuring the chemical, physical, and biological characteristics of water relative to standards for ecosystem and human health and safety. Monitoring helps identify pollution levels to control them. It is important because all life needs water, but many pollutants are invisible and can harm humans and environments. Proper monitoring uses tools and tests to evaluate factors like bacteria, nutrients, temperature, dissolved oxygen, turbidity, and pH that indicate a body of water's quality and ability to support life. Individual actions can help reduce pollution and improve water quality through practices like cleaning up debris, limiting paved surfaces, and reporting issues.