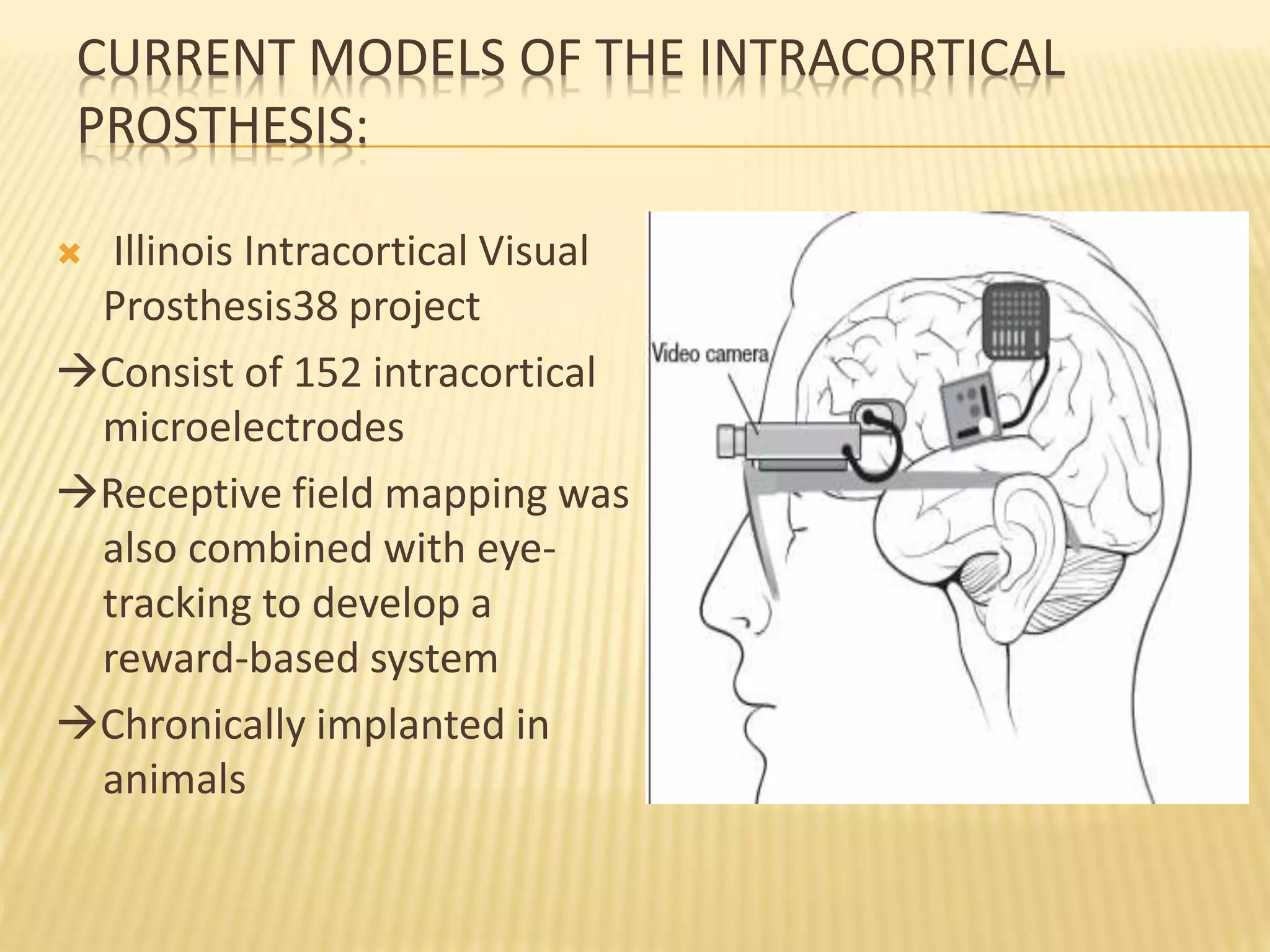
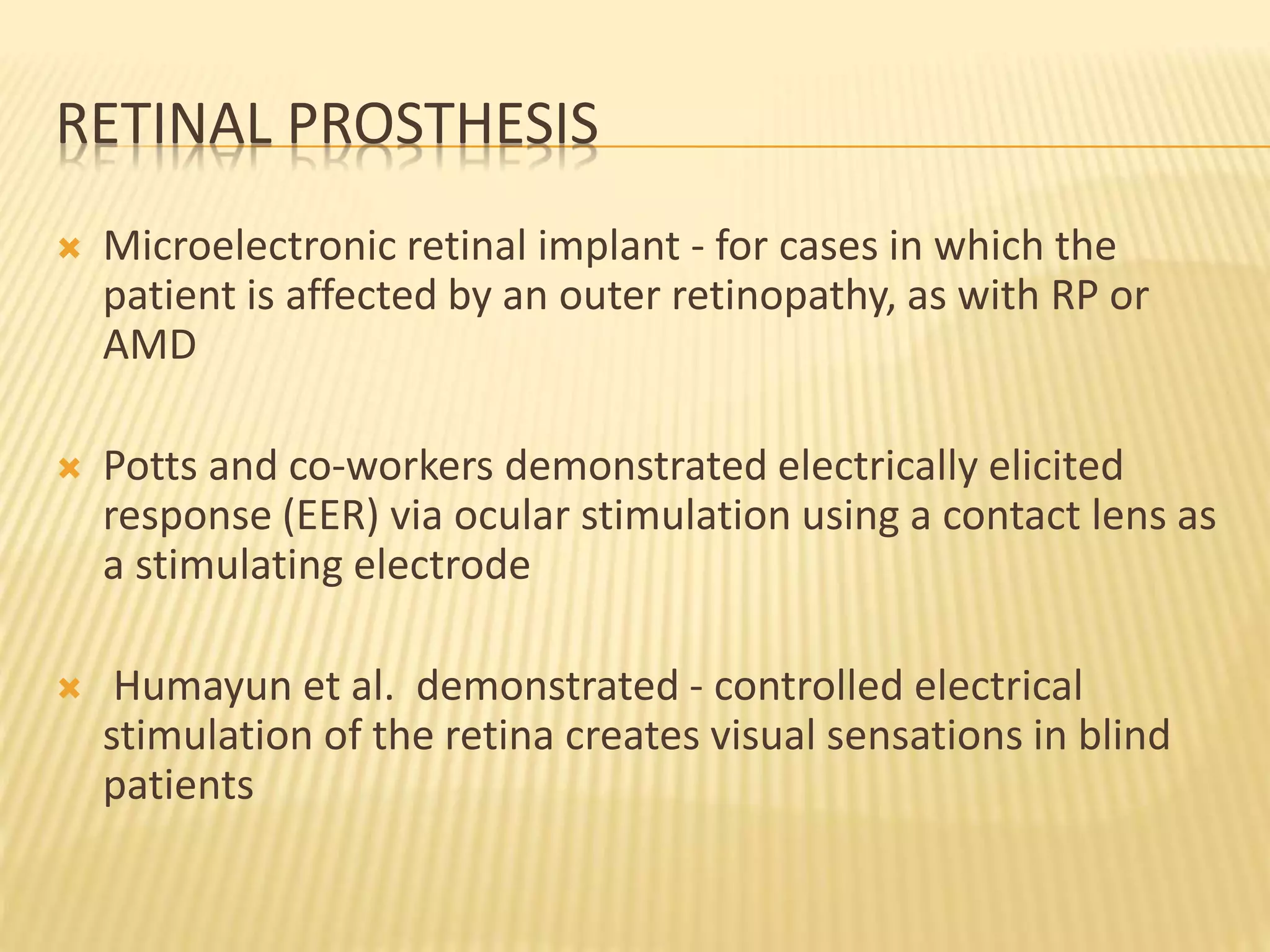
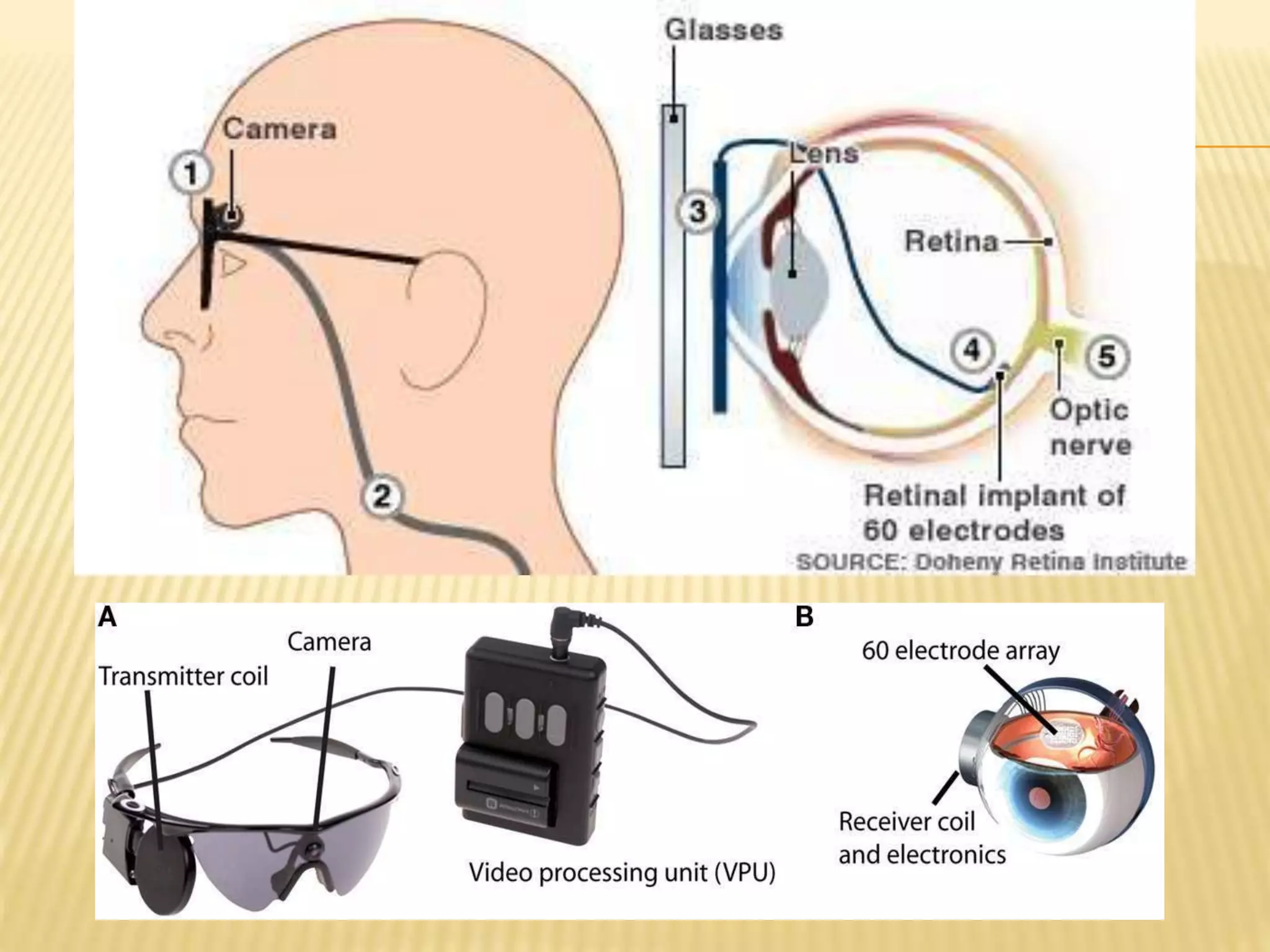
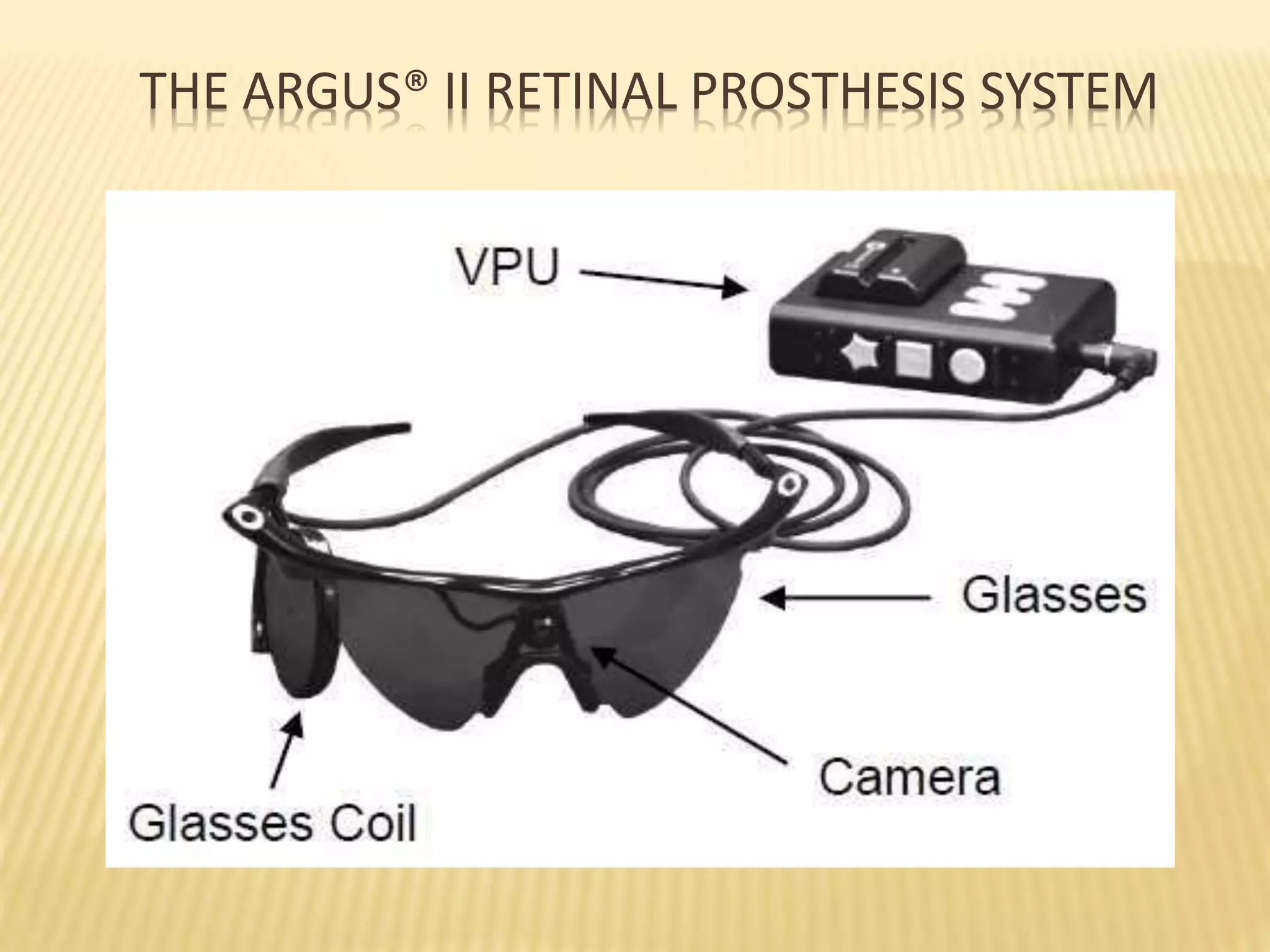
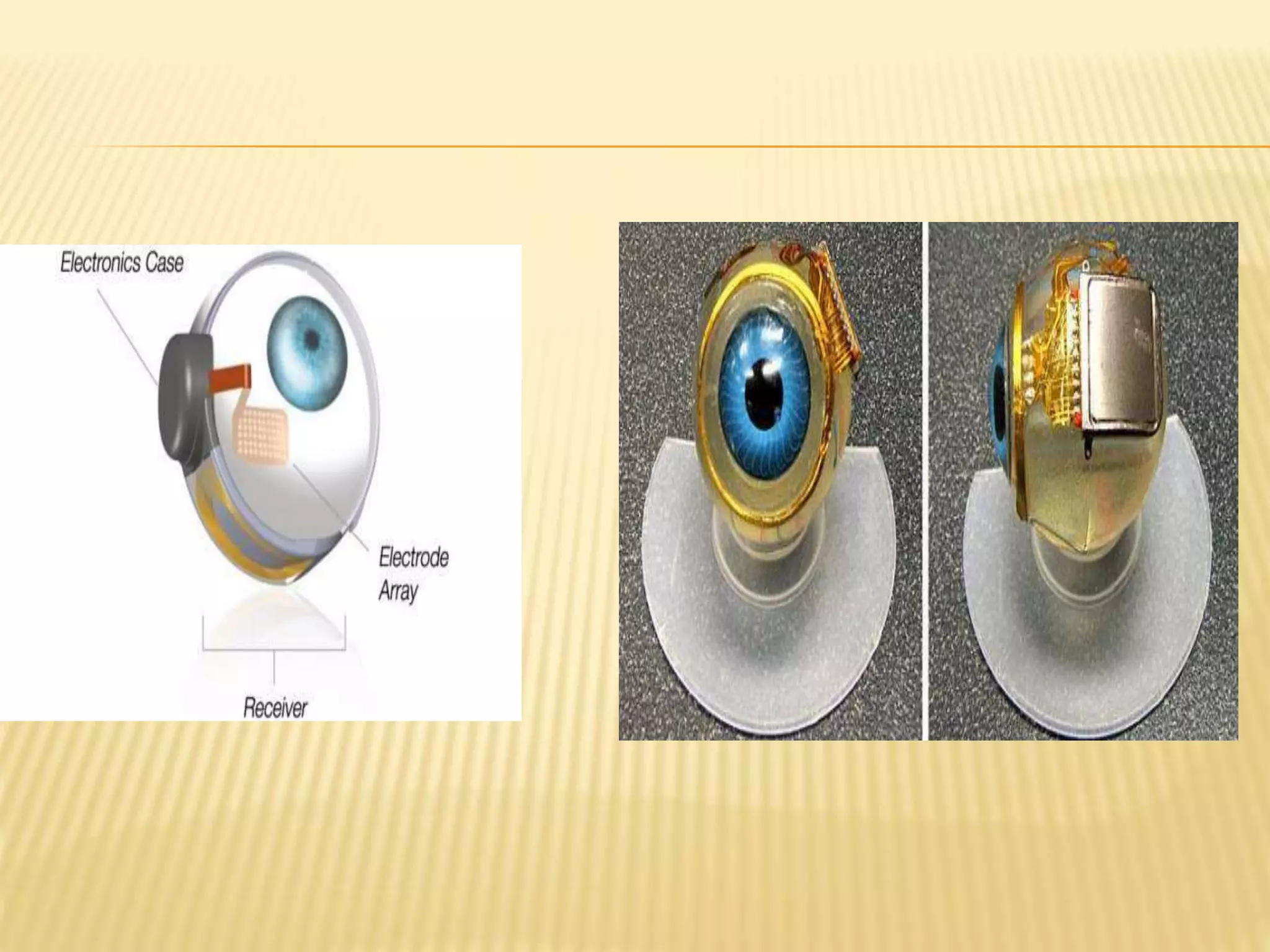
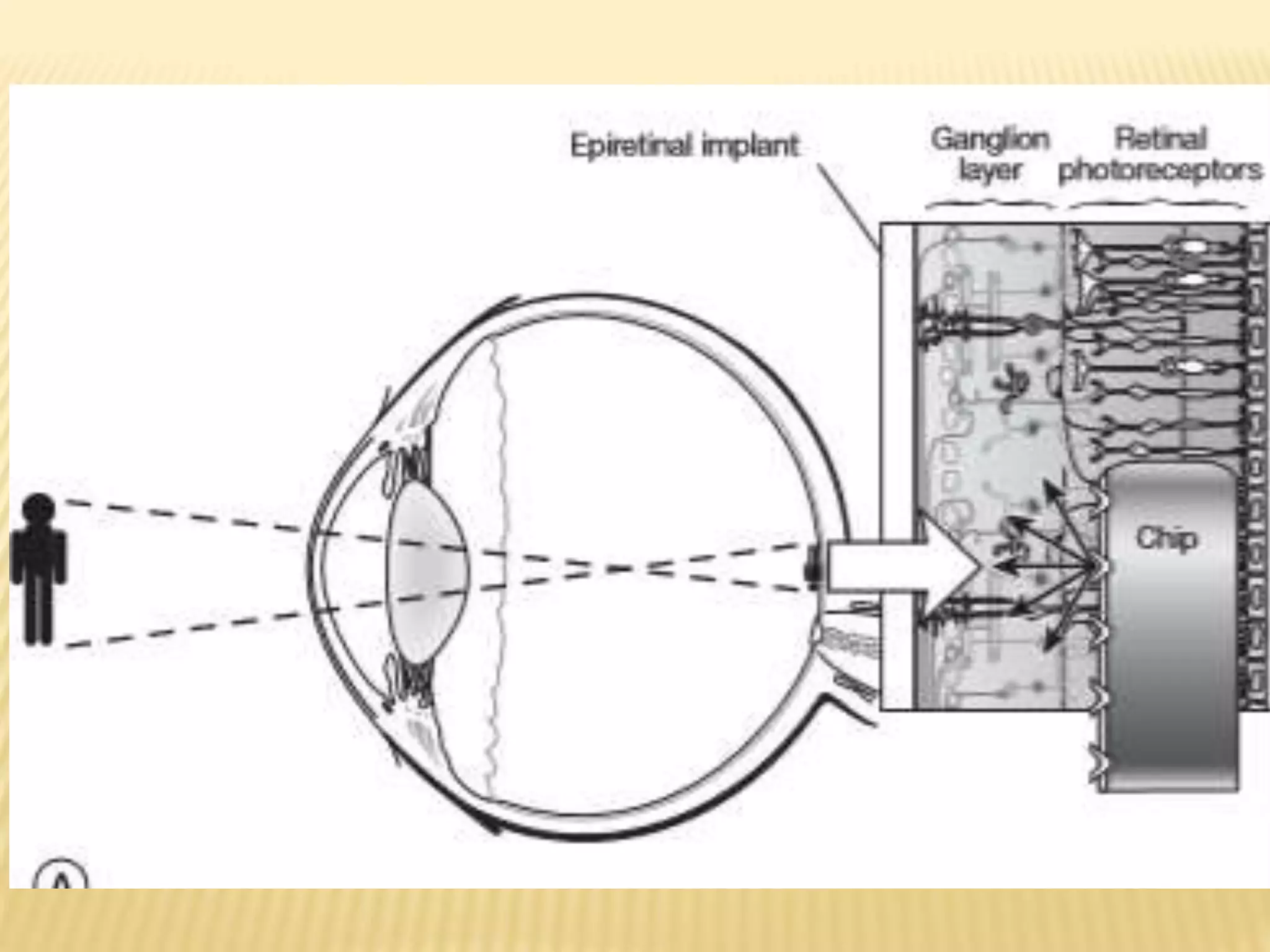
The document discusses visual prostheses, which are devices designed to restore vision through neuronal electrical stimulation at various locations along the visual pathway. It outlines different types of visual prostheses, including cortical, optic nerve, retinal, and suprachoroidal implants, along with their functionalities, advantages, limitations, and the developmental history from early concepts to recent advancements like the Argus II system. Additionally, it highlights ongoing efforts in India to develop more affordable retinal implants to improve accessibility for patients with visual impairments.