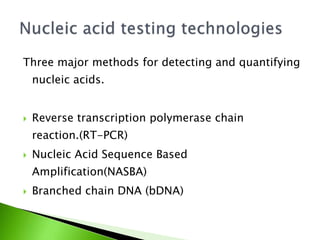
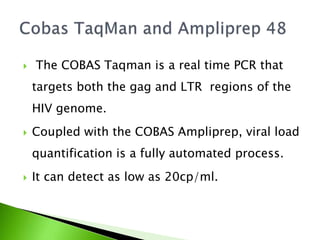
Viral load assays are changing due to advanced technology to better serve both developed and developing countries. There are two main types of viral load assays: nucleic acid testing (NAT) technologies that detect and quantify viral RNA, and non-nucleic acid testing (NNAT) technologies that detect viral enzymes and proteins as a correlate of viral RNA. Common NAT methods include reverse transcription polymerase chain reaction (RT-PCR), nucleic acid sequence based amplification (NASBA), and branched chain DNA (bDNA). RT-PCR is commonly used to quantify HIV RNA and determine viral load. Examples of commercial viral load tests include Amplicor HIV-1 monitor v1.5, Cobas Taqman, and Real time HIV-1