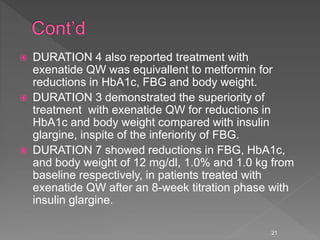
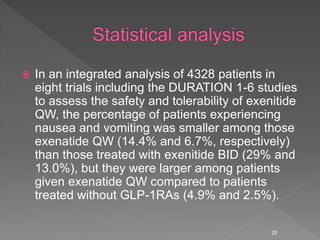
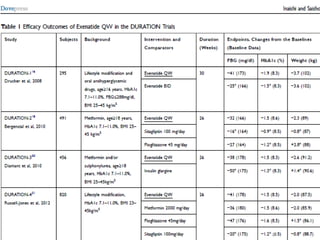
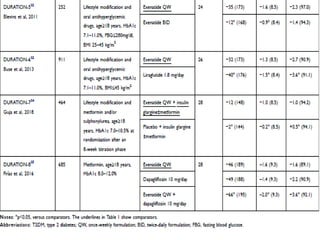
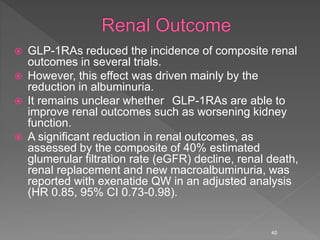
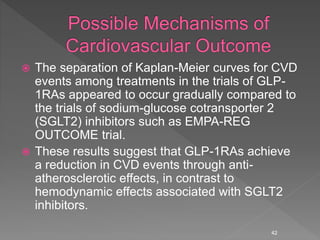
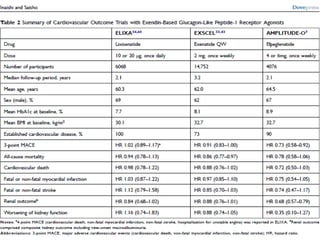
This document summarizes research on the efficacy and safety of the glucagon-like peptide-1 receptor agonist exenatide once weekly (QW) for the treatment of type 2 diabetes. It describes several clinical trials (DURATION studies) that evaluated exenatide QW and found it reduced HbA1c, fasting blood glucose, and body weight over 24-30 weeks. Adverse effects like nausea and diarrhea were less than other GLP-1RAs but injection site reactions were more common. Overall, the studies demonstrated the clinical efficacy of exenatide QW for type 2 diabetes treatment.