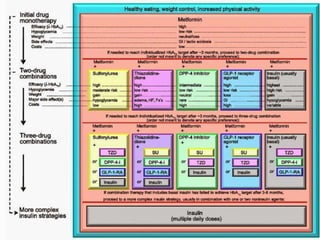
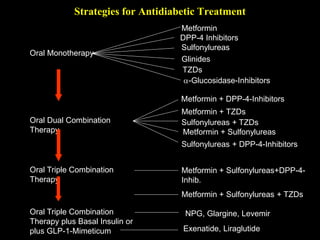
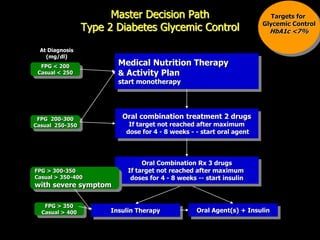
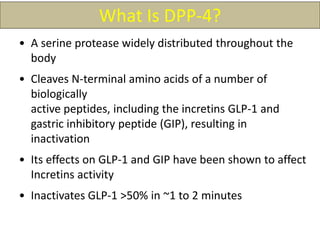
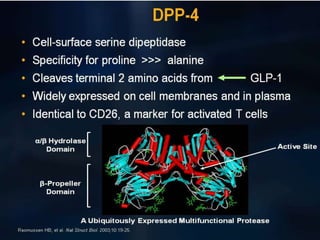
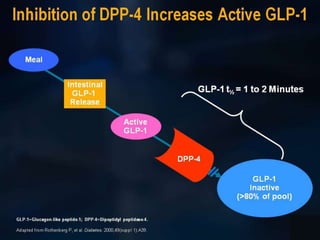
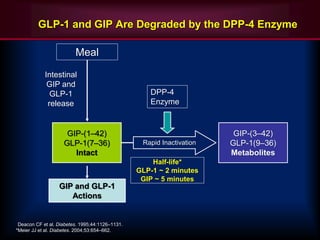
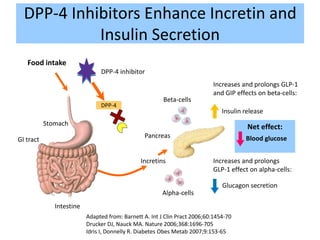
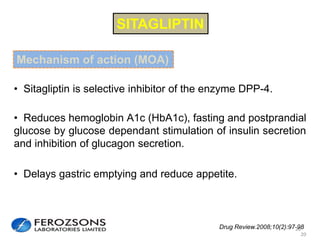
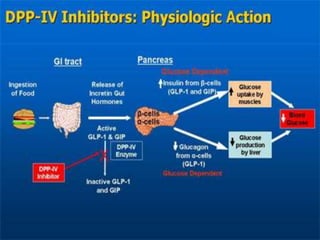
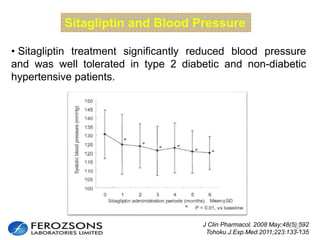
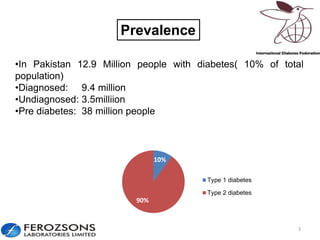
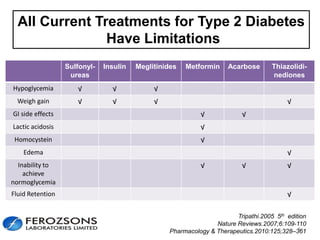
The document provides an overview of diabetes treatment updates, focusing on the prevalence of diabetes in Pakistan and management strategies for type 2 diabetes. It highlights the role of Sitagliptin as a DPP-4 inhibitor, detailing its mechanism of action, benefits, and clinical findings, including its effect on glycemic control and tolerability. Additionally, it covers treatment combinations, the role of incretins, and therapeutic guidelines from the ADA.



![No Single Class of Oral Antihyperglycemic
Monotherapy Targets All Key Pathophysiologies
Alpha-
Glucosidase
Inhibitors1,2
Meglitinide
s3 SUs4,5 TZDs6,7
Metformi
n8
DPP-4
Inhibitors
Insulin
deficiency
Insulin
resistance
Excess
hepatic
glucose
output
MajorPathophysiology's
1. Glyset [package insert]. New York, NY: Pfizer Inc; 2004. 2. Precose [package insert]. West Haven, Conn: Bayer; 2004.
3. Prandin [package insert]. Princeton, NJ: Novo Nordisk; 2006. 4. Diabeta [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2007.
5. Glucotrol [package insert]. New York, NY: Pfizer Inc; 2006. 6. Actos [package insert]. Lincolnshire, Ill: Takeda Pharmaceuticals; 2004.
7. Avandia [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
8. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2004.
Intestinal
glucose
absorption
](https://image.slidesharecdn.com/sitagliptin-140601120632-phpapp02/85/Sitagliptin-2015-6-320.jpg)