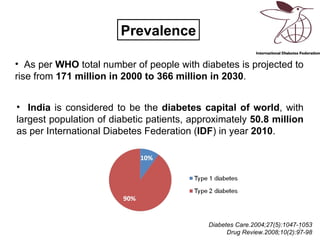
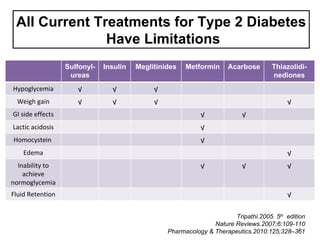
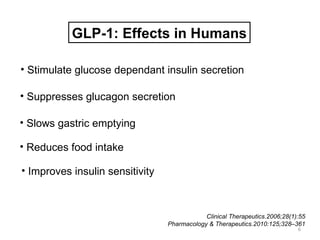
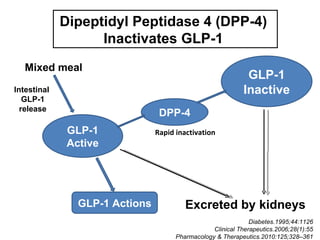
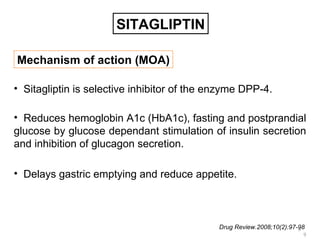
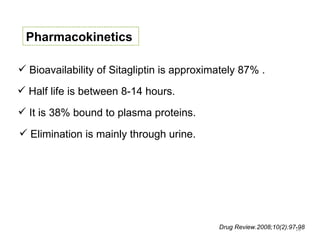
This document discusses the treatment of diabetes with a focus on the drug Sitagliptin. It provides details on Sitagliptin's mechanism of action as an inhibitor of DPP-4, pharmacokinetics, clinical efficacy in reducing HbA1c and glucose levels, safety profile, recommended dosage, drug interactions, and regulatory approval history. Sitagliptin represents an effective newer treatment option for type 2 diabetes with a good tolerability profile.