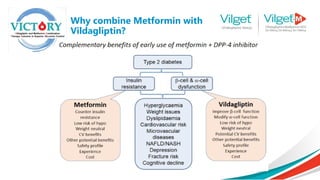
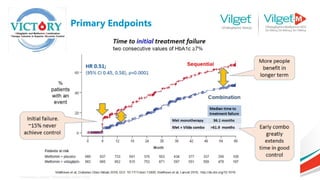
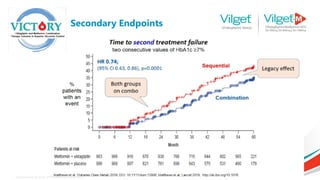
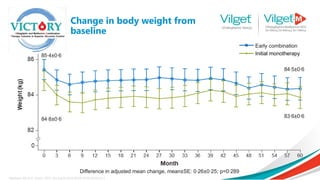
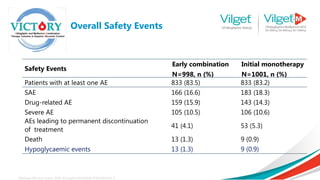
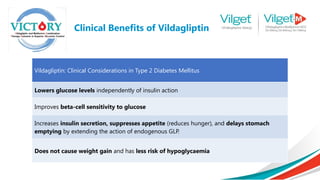
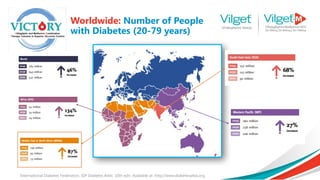
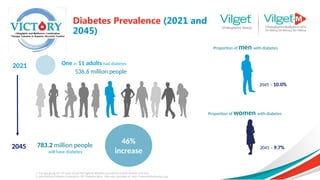
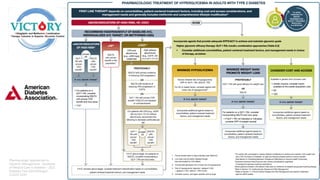
The document discusses the global rise in diabetes prevalence, particularly highlighting that by 2045, an estimated 783.2 million people will have diabetes, with alarmingly high rates in Pakistan. It outlines the efficacy of vildagliptin and metformin in improving glycemic control, emphasizing vildagliptin's effectiveness both as monotherapy and in combination with other treatments like metformin and insulin. The findings suggest that vildagliptin not only lowers HbA1c levels significantly but also reduces the risks of diabetes-related complications.










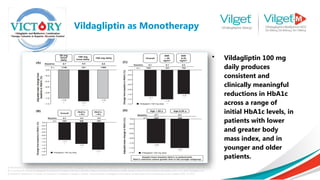




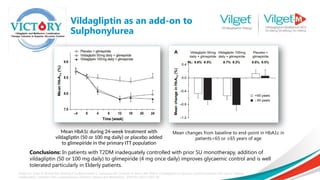
![ Primary efficacy variable was change
in HbA1c from baseline to week 24
endpoint
Secondary efficacy variables included
change from baseline at the week 24
endpoint in FPG, body weight and
fasting lipids
Results: Both vildagliptin plus high- and
low-dose metformin combination
therapy demonstrated statistically
significant superiority in lowering HbA1c
compared with vildagliptin and
metformin monotherapy
The greatest reductions were seen in the vildagliptin
and metformin combination groups, and the largest
change was in the vildagliptin plus high-dose
metformin combination therapy group [adjusted
mean (SE) change from baseline: 1.8% (0.06%)].
Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment‐
naive patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2009 May;11(5):506-15.
Results: Vildagliptin Plus
Metformin Therapy](https://image.slidesharecdn.com/vilgetpresentation1-241009080316-45b0ea63/85/Vilget-Presentation-1-pptx-Diabetes-emergency-22-320.jpg)

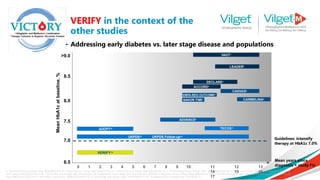
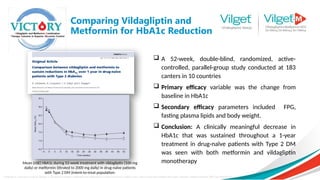
![Consistent effectiveness between RCT and real-life data observed with MET + vildagliptin
while the SU-combination in real-life setting has lower effectiveness vs. RCTs
Metformin + Sulphonylureas
Metformin + Vildagliptin
• Vildagliptin efficacy is consistent
between RCTs and real life (EDGE)
• Efficacy with SU appears to be
diminished in real life versus RCTs
EDGE
RCT
Ahrén B et al. Diabetologia 2014 DOI 10.1007/s00125-014-3222-z
Data were pooled from 5 clinical trials carried out in 4480 patients [Vilda, N=2788 or SUs, N=1692 (glimepiride, n=1259; gliclazide, n=433)] and
compared with a large sample of patients extracted from the EDGE study (Vilda, n=7002 or SUs, n=3702). Linear regression analyses were
performed between the baseline HbA1c and the change in HbA1c (ΔHb1c) after 24 weeks.
Real Life Data](https://image.slidesharecdn.com/vilgetpresentation1-241009080316-45b0ea63/85/Vilget-Presentation-1-pptx-Diabetes-emergency-24-320.jpg)