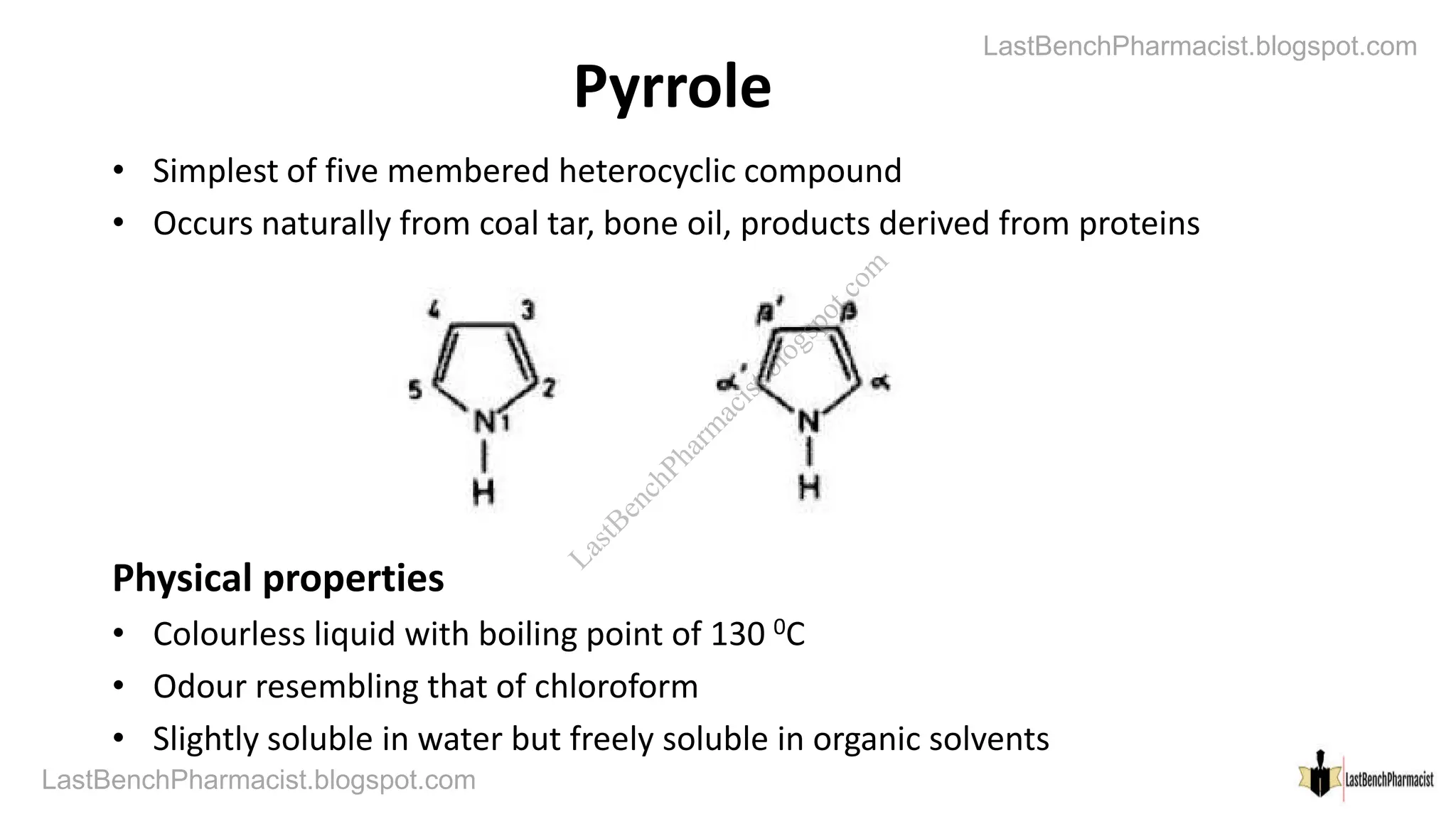
The document discusses pyrrole, including its chemistry, synthesis, reactions, and importance. Pyrrole contains sp2 hybridized carbons forming a planar pentagonal ring with one electron from each carbon and two from the heteroatom contributing to aromaticity. It is synthesized from furan, mucate, and 1,4-diketones. Pyrrole undergoes electrophilic substitution readily and is present in natural compounds like heme and chlorophyll. Medicinal compounds are also derived from pyrrole chemistry.