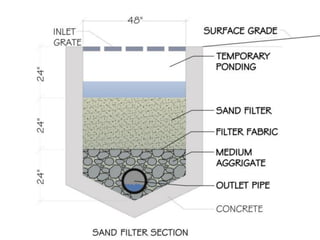
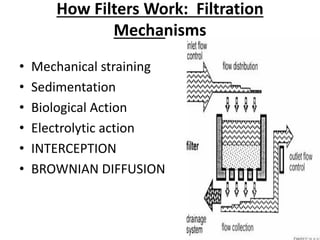
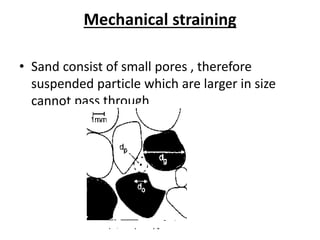
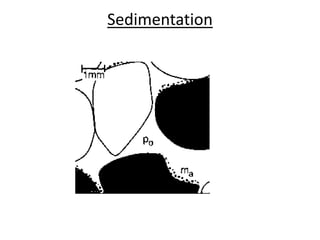
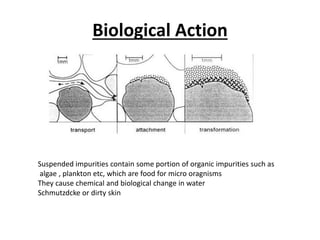
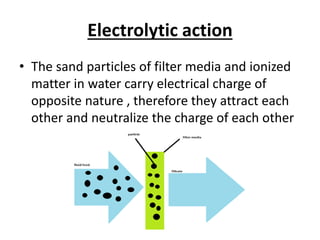
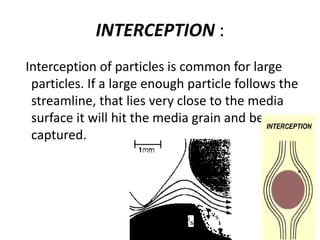
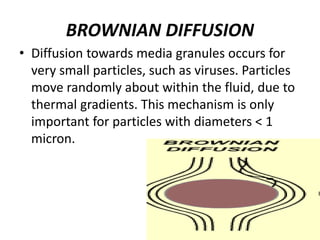
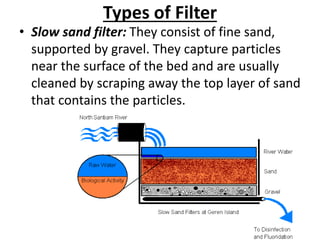
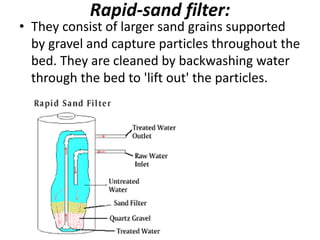
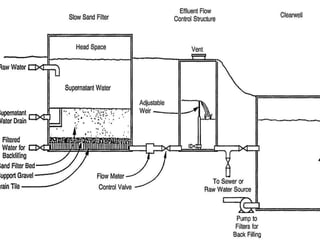
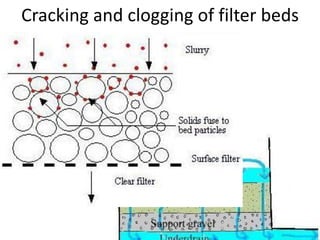
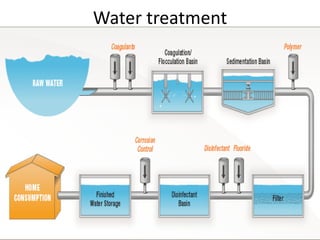
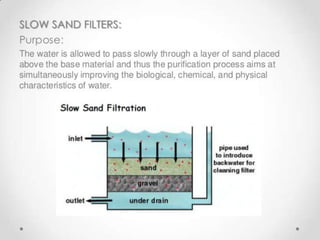
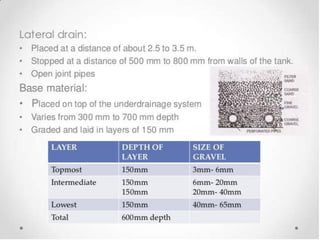
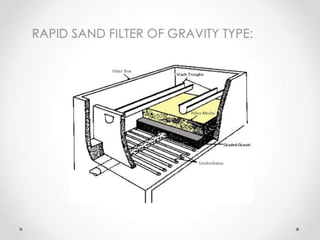
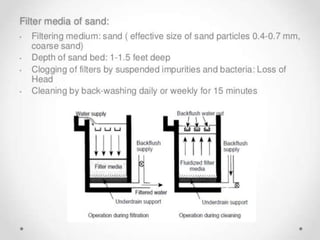
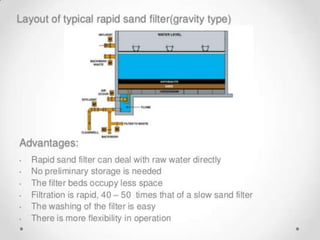
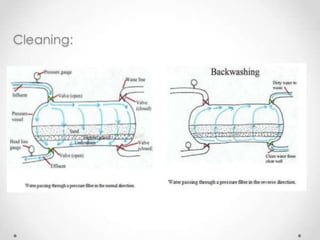
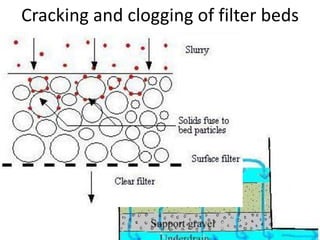
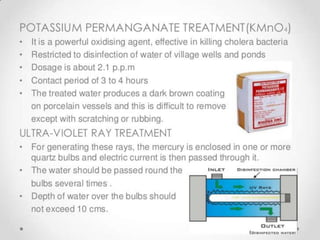
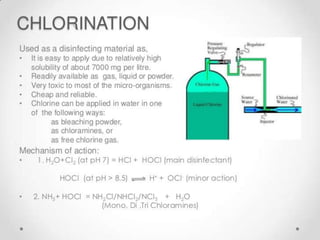
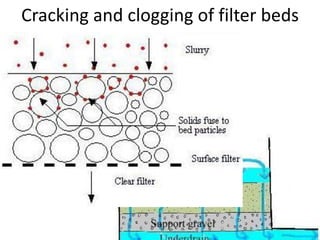
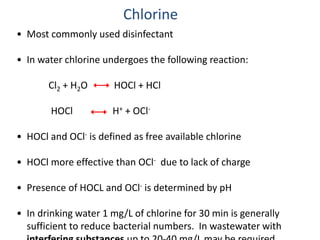
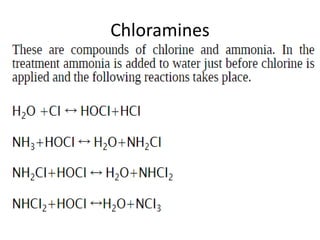
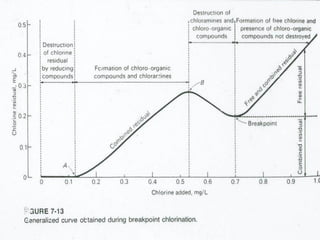
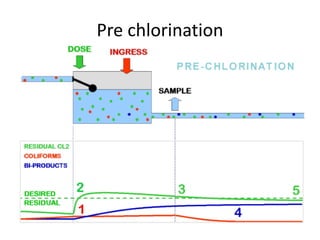
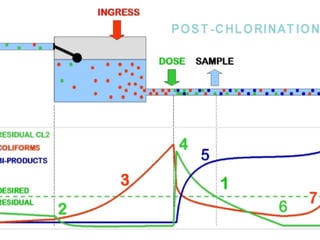
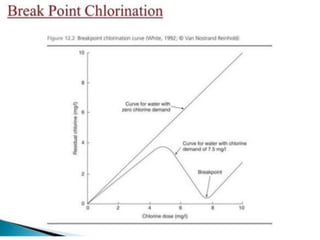
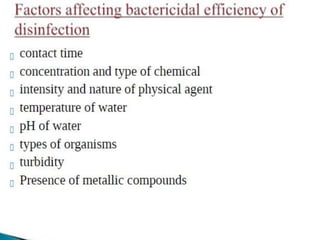
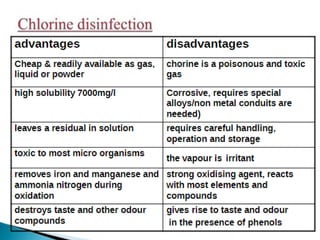
This document discusses various types of water filtration methods and processes. It describes different filter media like sand, gravel, anthracite coal and their characteristics. It also explains mechanisms involved in filtration like mechanical straining, sedimentation, biological action etc. Further, it provides details about slow sand filters and rapid sand filters, including their construction, operation, efficiency and common issues like cracking, clogging, mud ball formation etc. The document also covers various disinfection methods used in water treatment like chlorination, chloramines, bleaching powder etc.