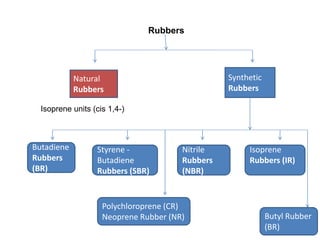
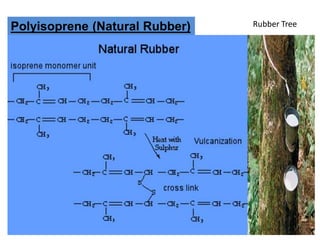
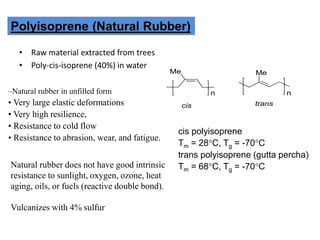
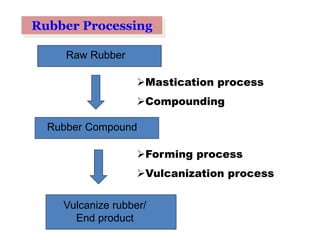
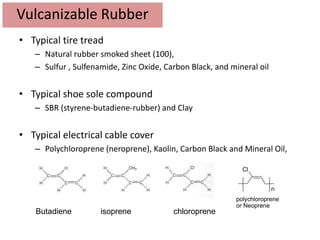
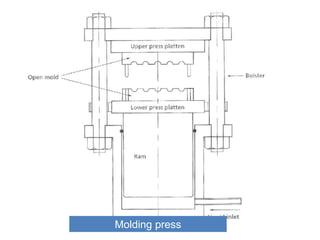
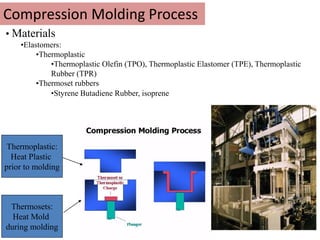
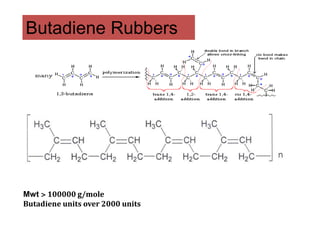
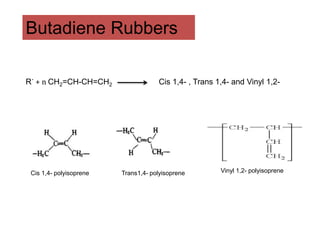
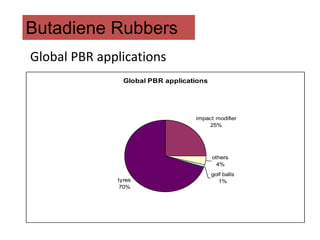
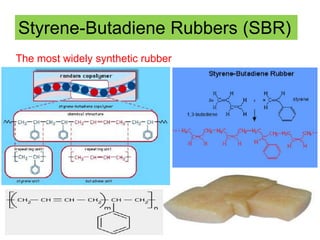
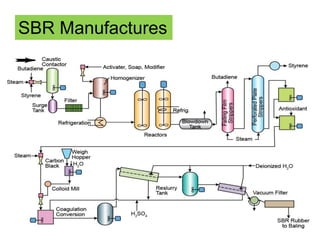
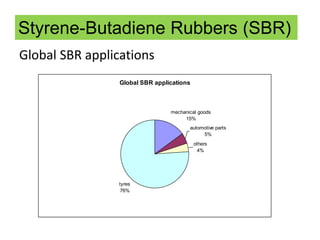
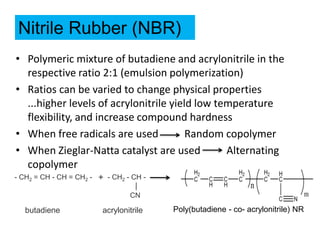
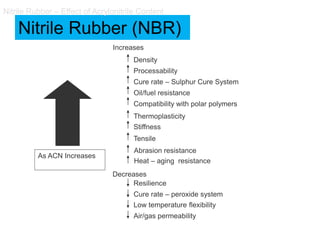
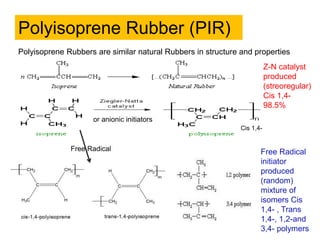
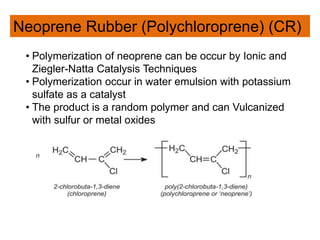
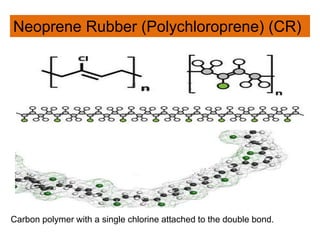
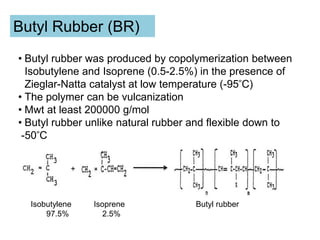
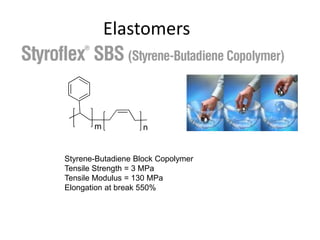
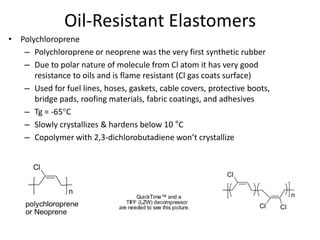
The document provides an overview of synthetic rubbers, including their types, properties, production methods, and applications. It discusses various rubber types such as isoprene, nitrile, styrene-butadiene, and neoprene, focusing on their manufacturing processes, compounding ingredients, and end uses. Key concepts like vulcanization and rubber processing techniques are also explained, highlighting the significance of additives and modifiers in enhancing rubber performance.