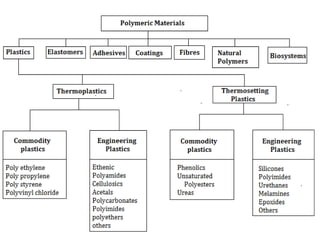
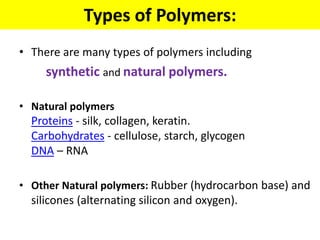
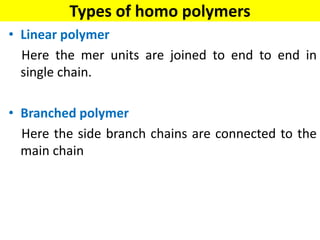
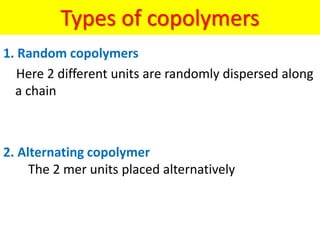
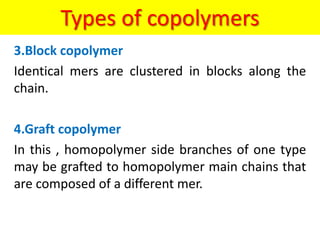
The document provides an overview of non-metallic materials, focusing on various types of polymers, their properties, structures, and applications. It highlights key polymers such as polyethylene, polypropylene, polystyrene, and polyvinylchloride, along with engineering ceramics and composites. Additionally, it discusses polymerization methods, classifications of polymers, and the significance of natural and synthetic polymers in various applications.