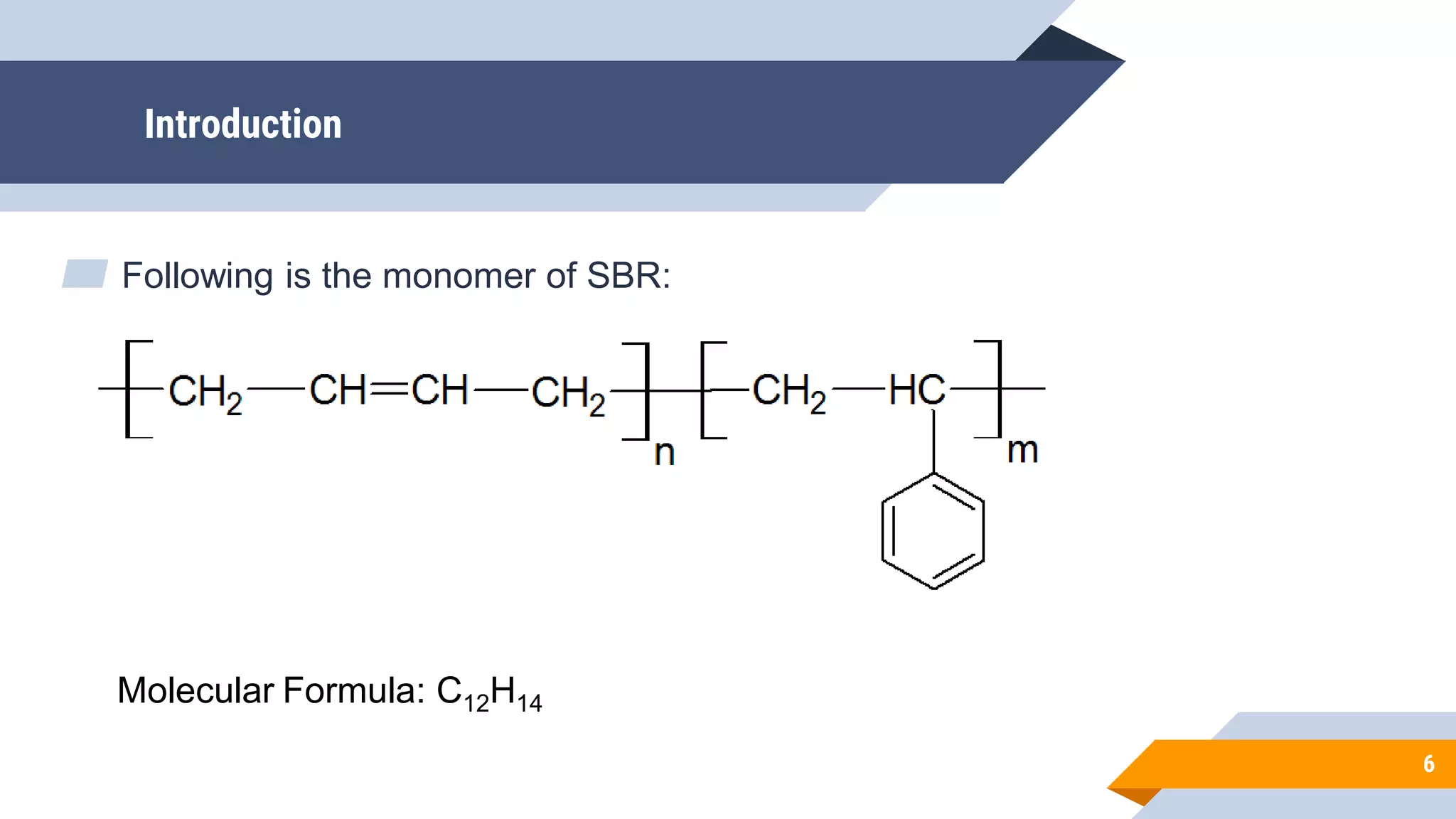
The document provides an overview of styrene-butadiene rubber (SBR), detailing its introduction, synthesis methods, applications, and properties. SBR, a synthetic rubber developed during WWII, is widely used in tire manufacturing and other applications due to its good abrasion resistance and varying properties based on styrene/butadiene ratios. It discusses both emulsion and solution polymerization processes, highlighting the importance of initiators and molecular weight control.