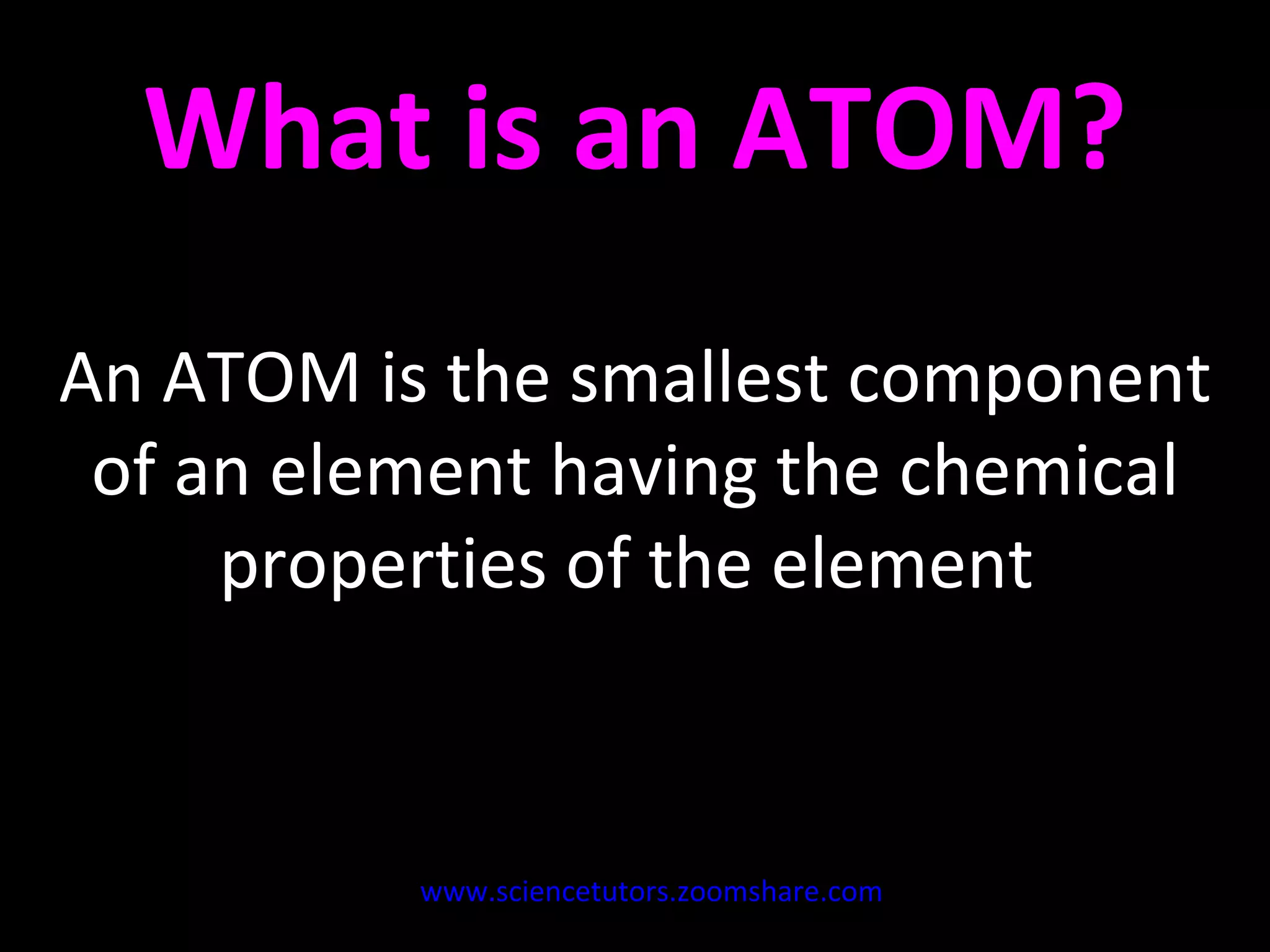
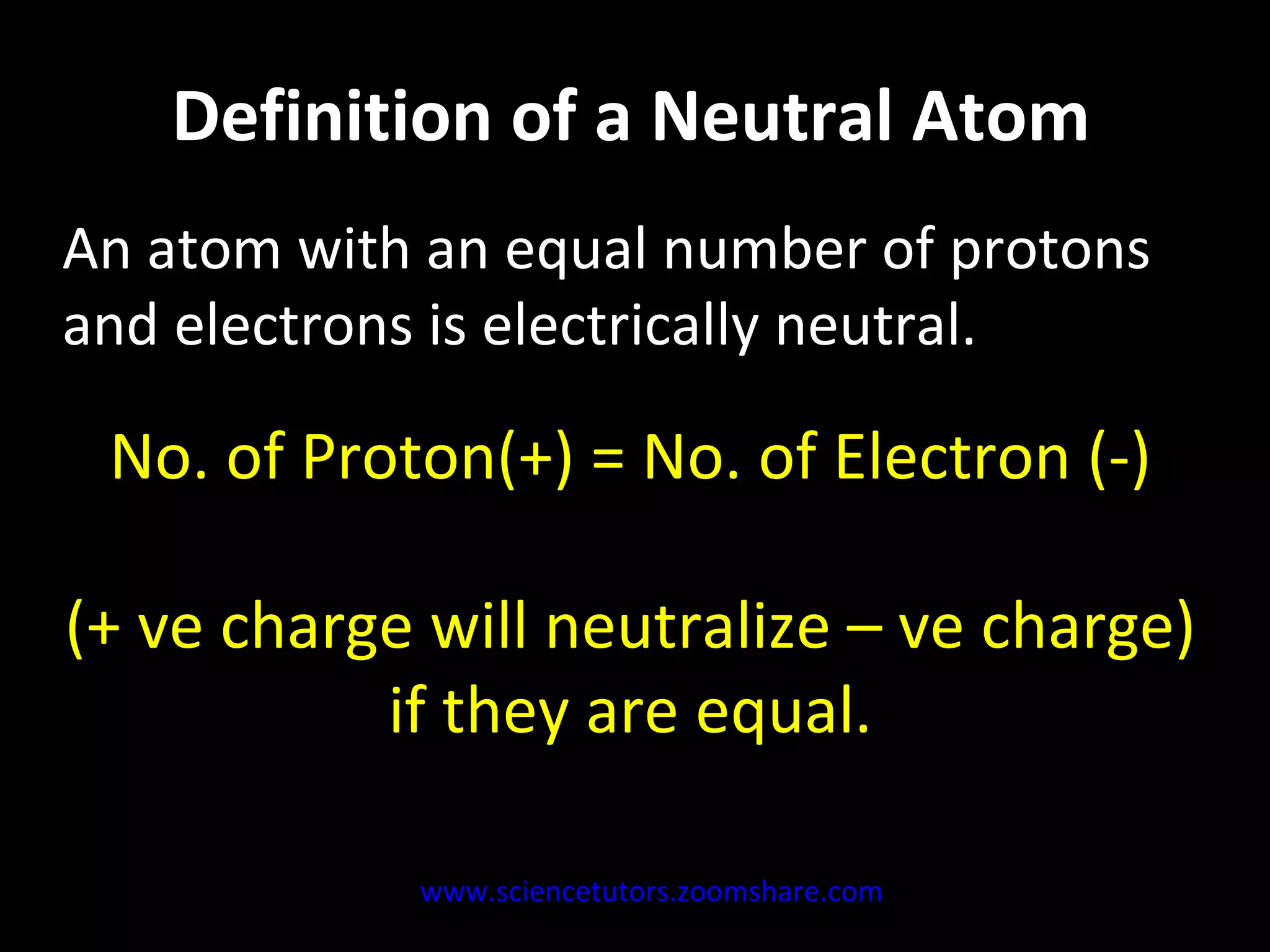
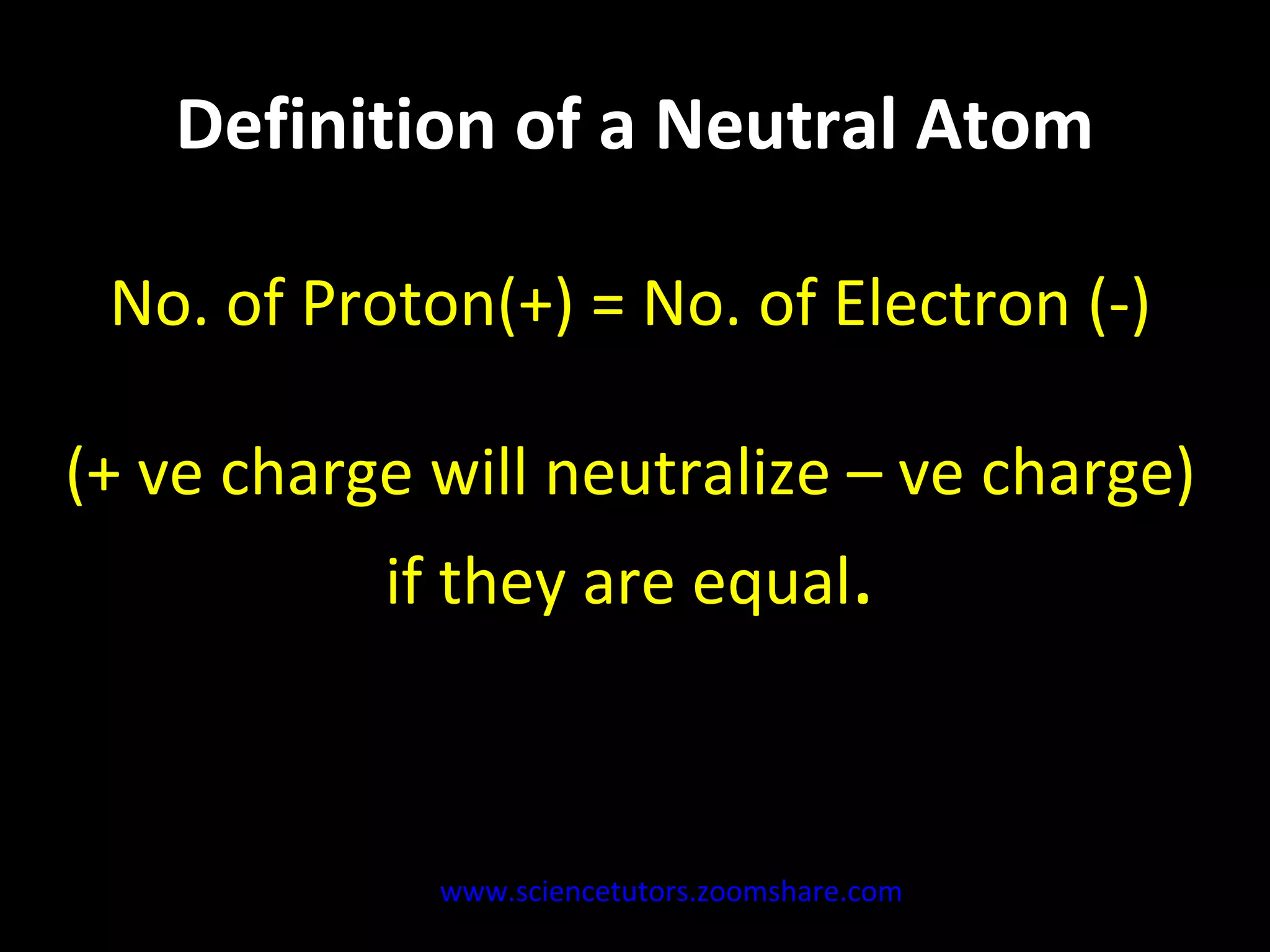
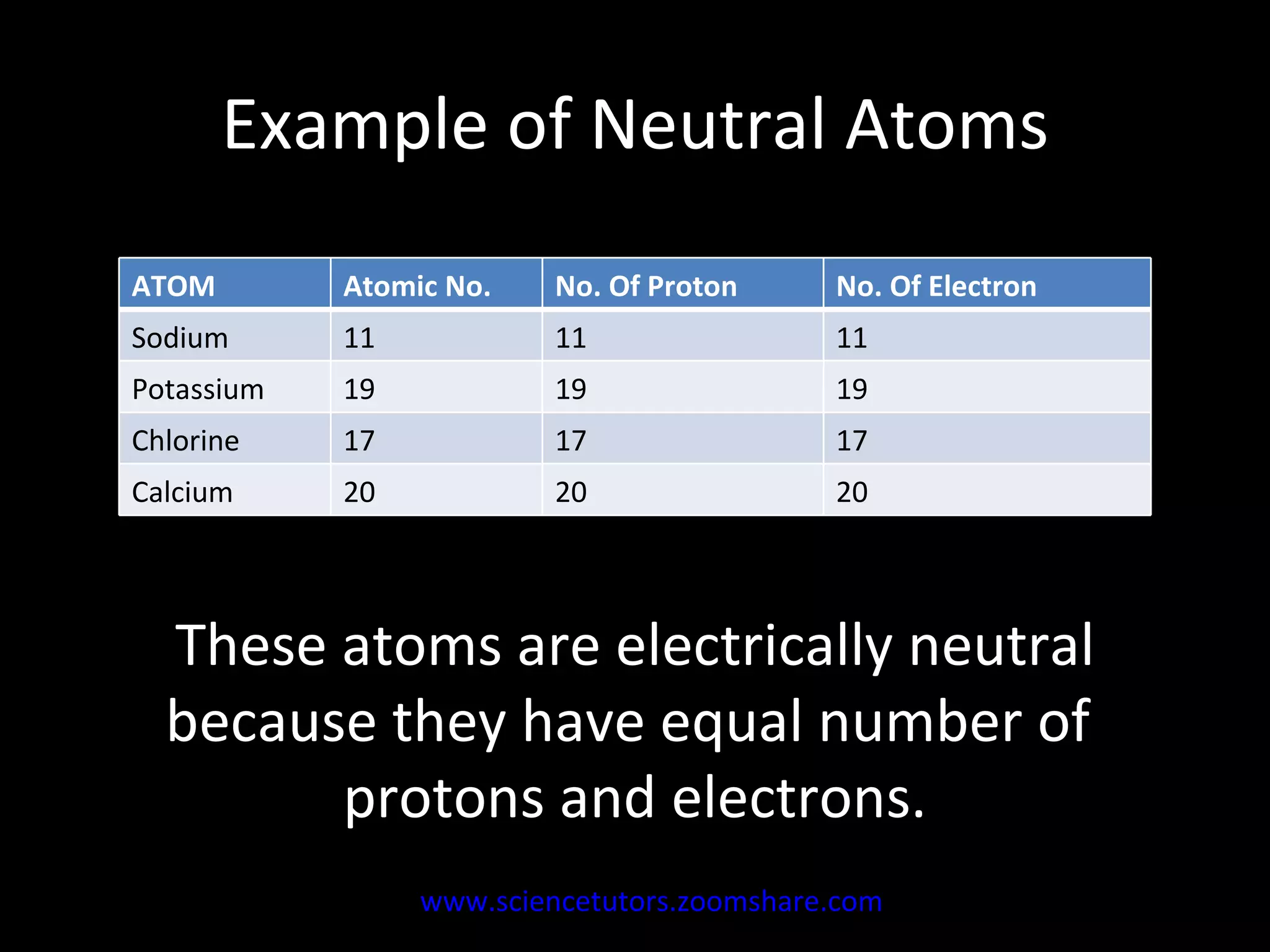
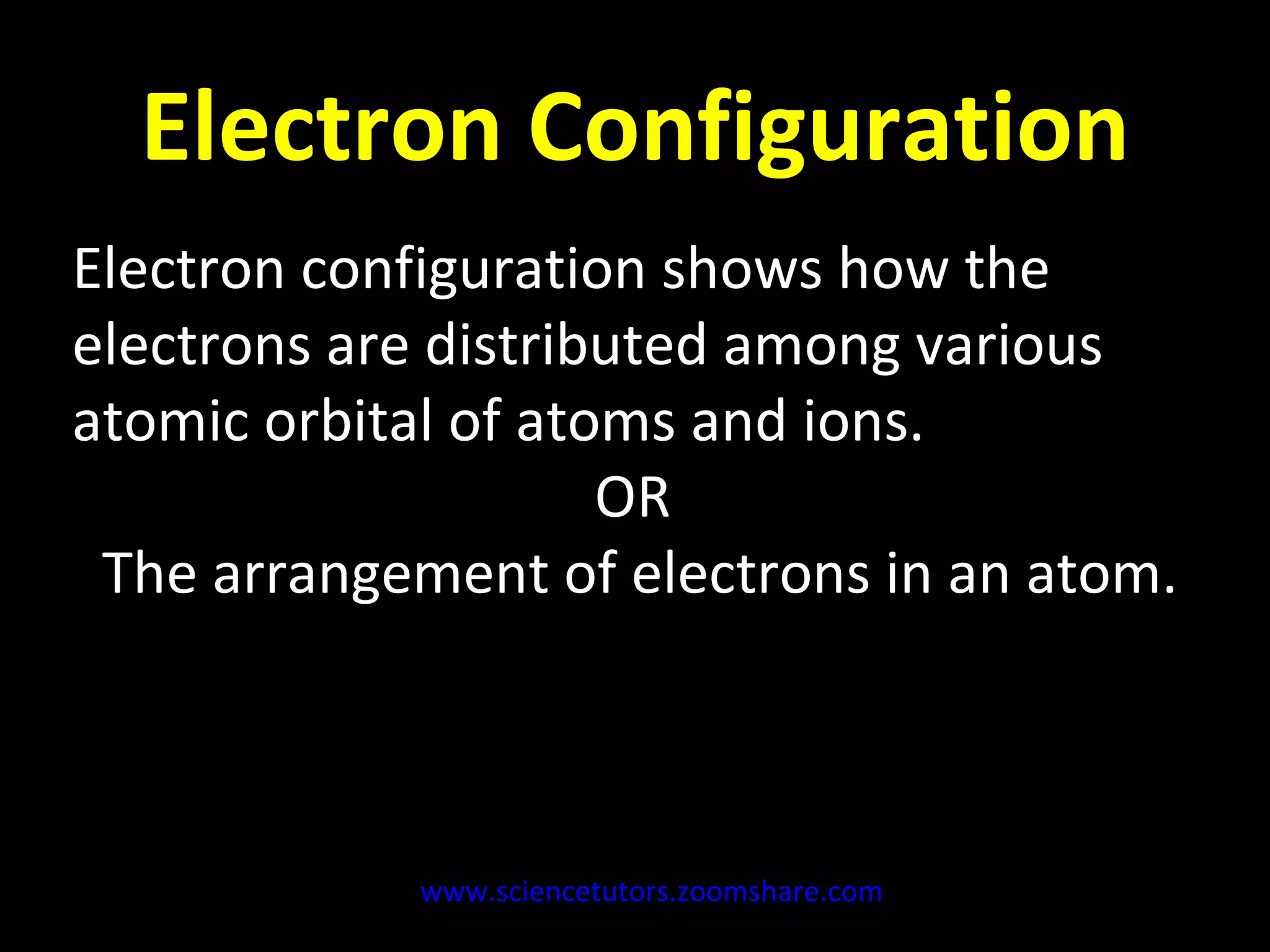
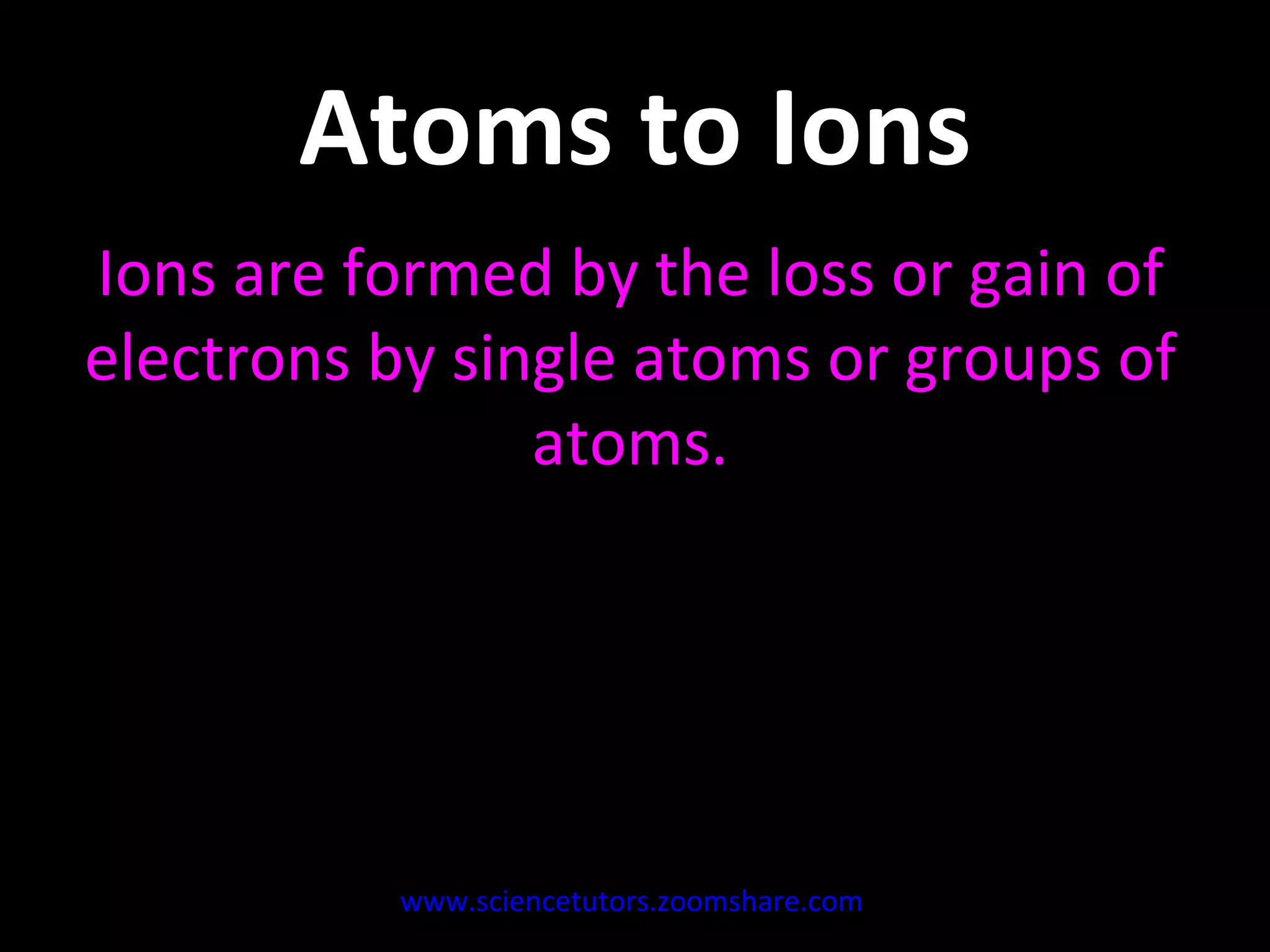
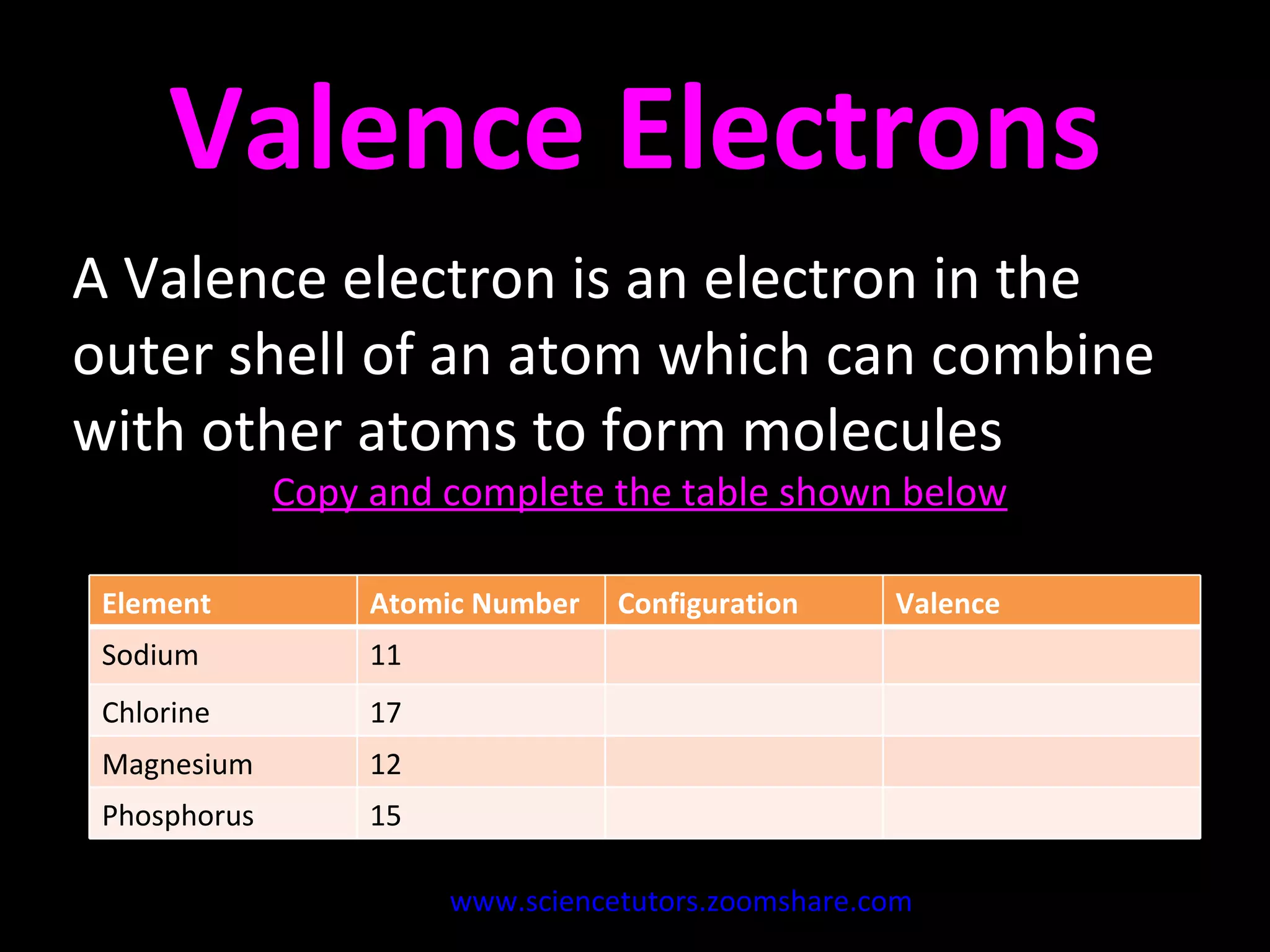
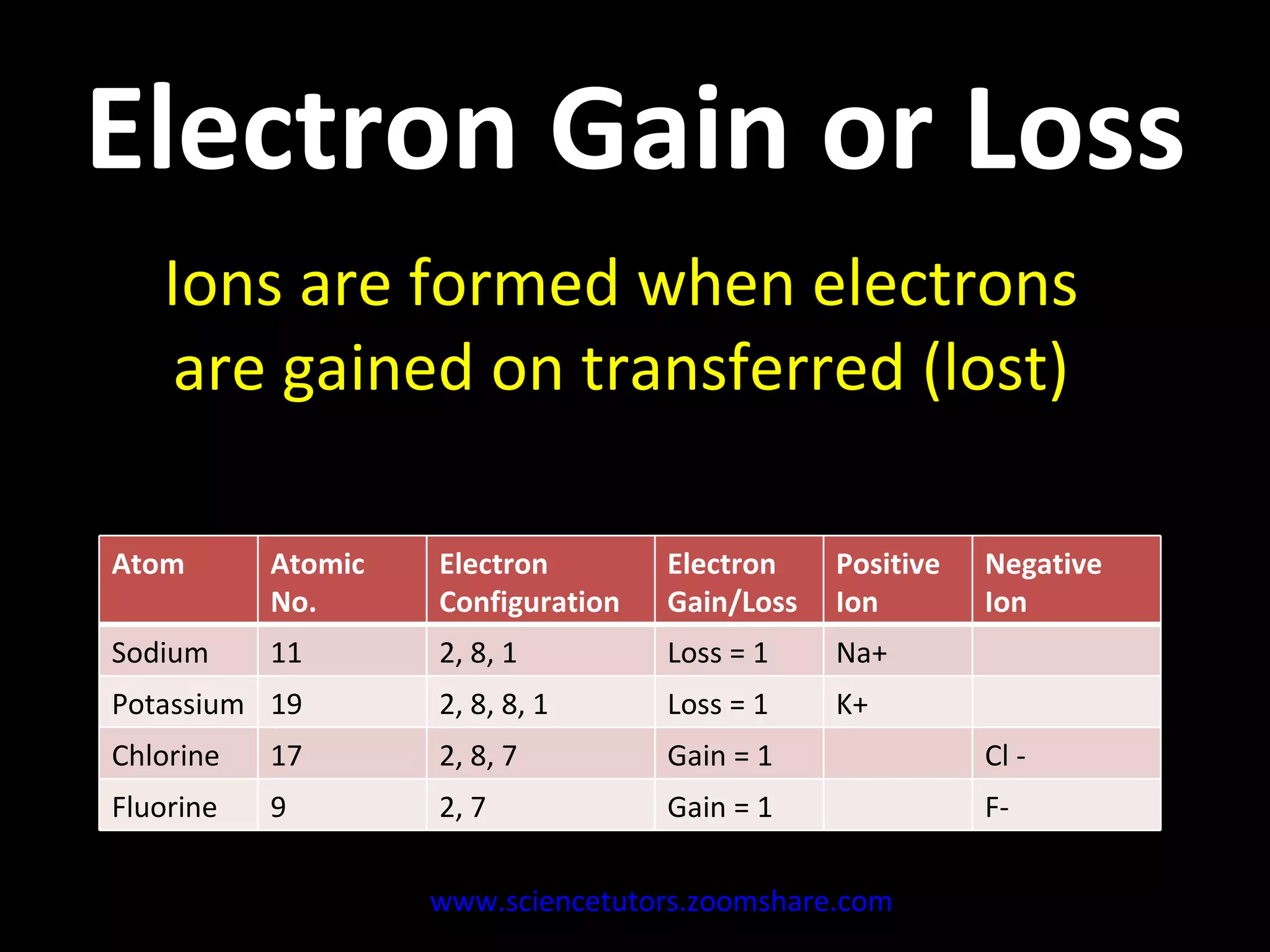
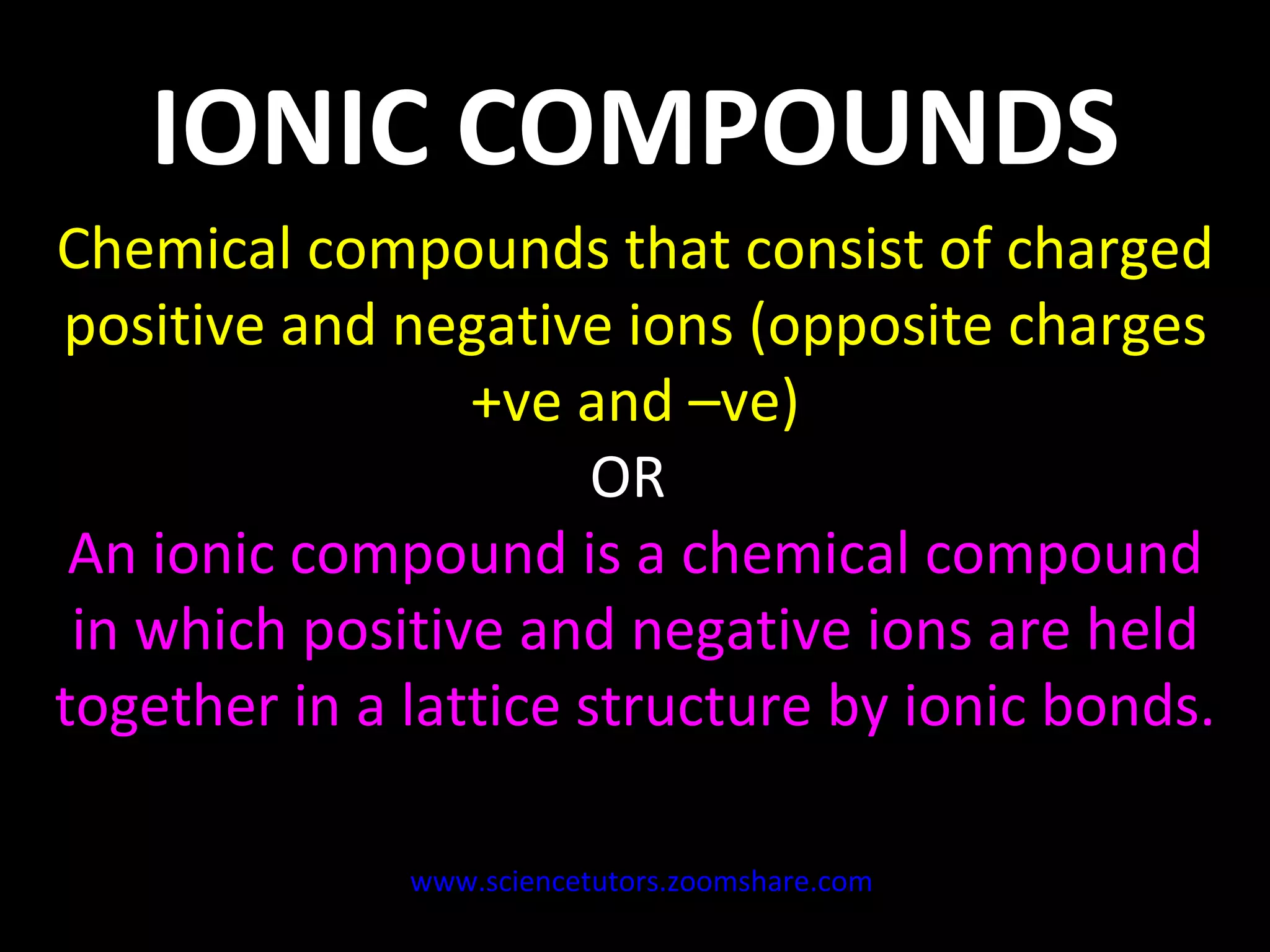
The document discusses the chemistry of atoms and ions. It defines atoms as the smallest component of an element, and defines ions as atoms that have gained or lost electrons, giving them a positive or negative charge. Neutral atoms have an equal number of protons and electrons, while ions form when atoms gain or lose valence electrons. Ionic compounds consist of positive and negative ions held together in a lattice by ionic bonds. Ionic compounds are generally solids with high melting points and conduct electricity due to the ions.