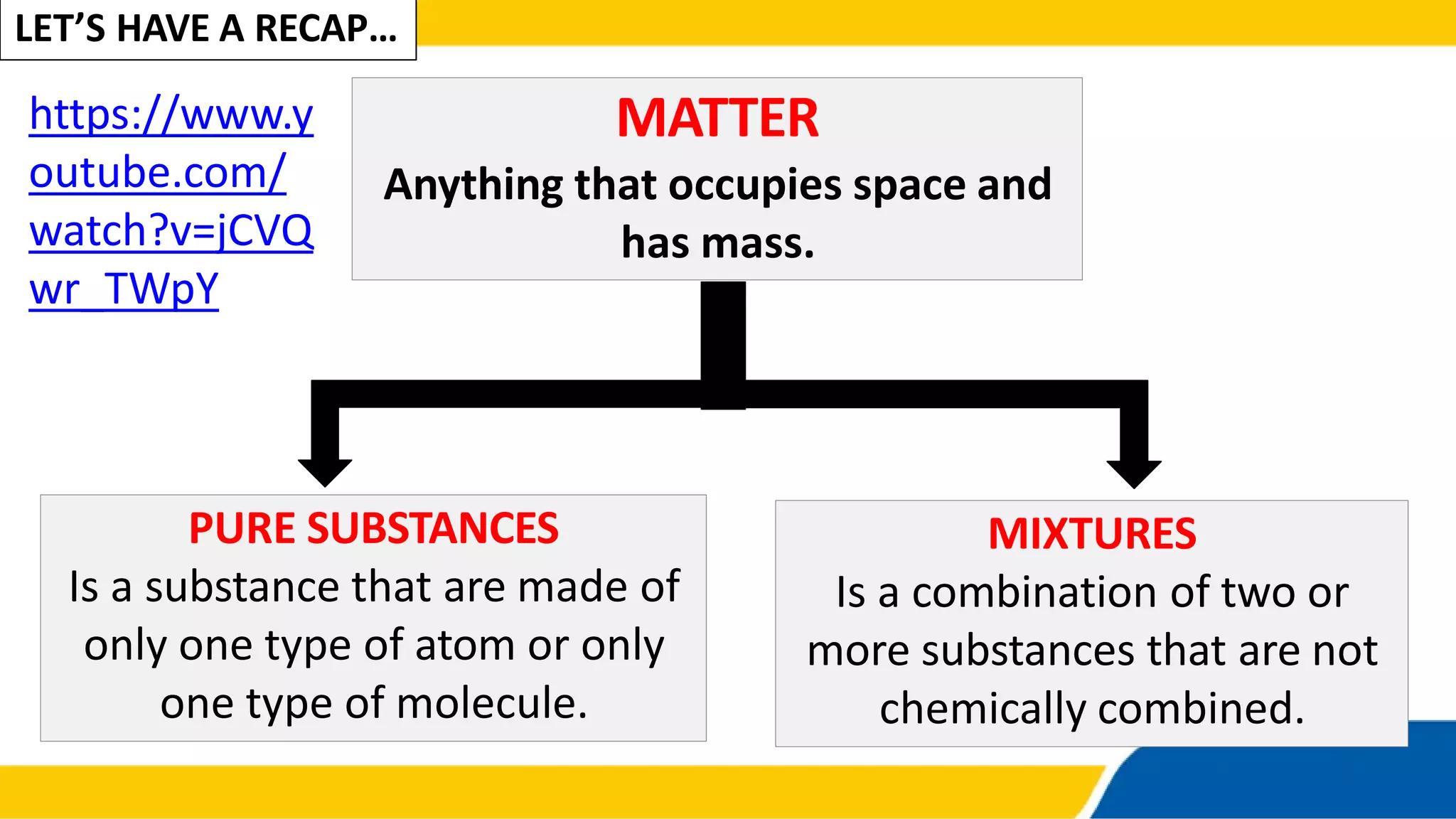
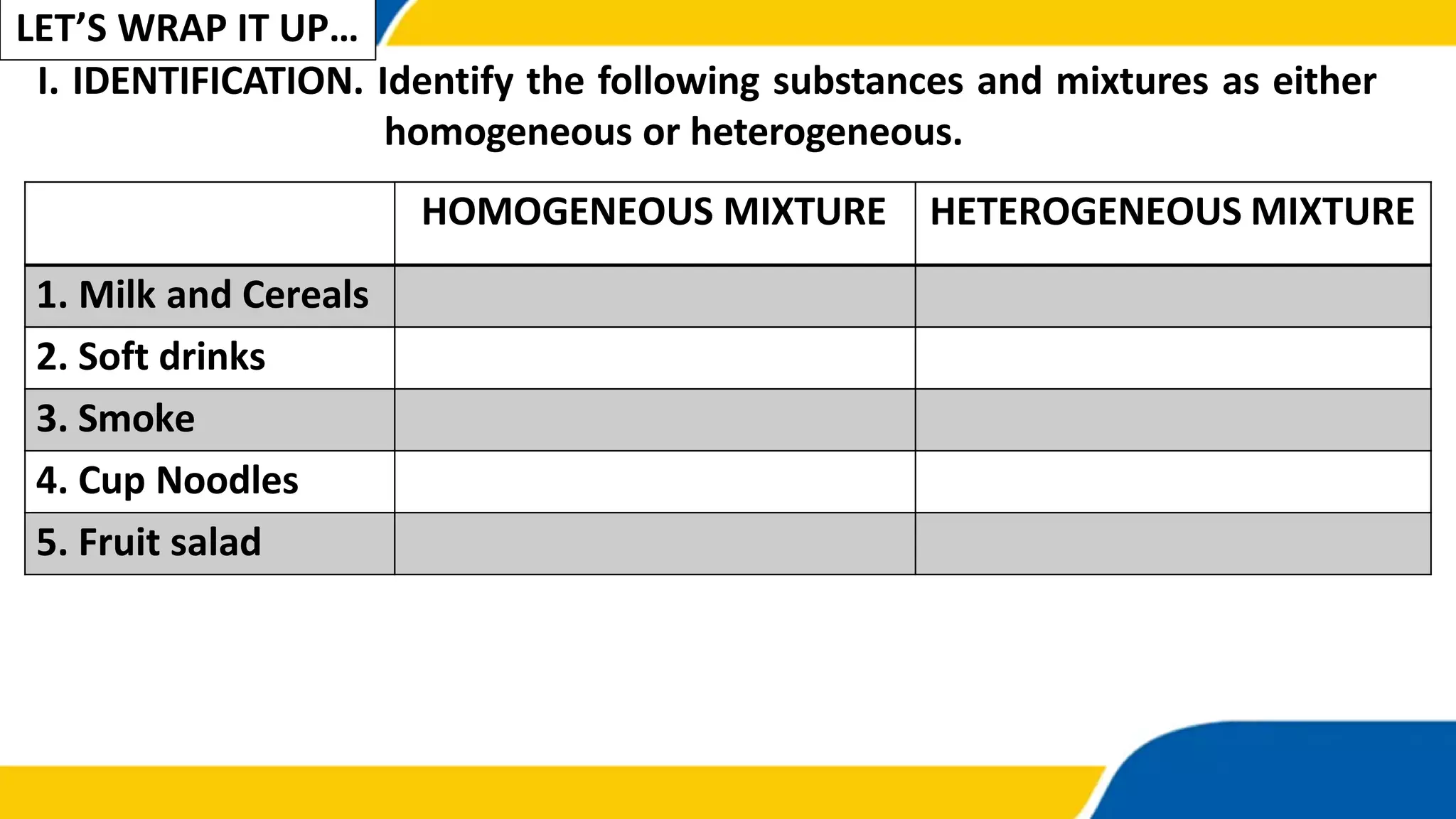
This document discusses types of mixtures. It defines a mixture as a combination of two or more substances that are not chemically combined. Mixtures are classified as either homogeneous or heterogeneous. A homogeneous mixture has components that are uniformly distributed and has uniform properties throughout. A heterogeneous mixture's components are not uniformly distributed and the mixture has varying properties in different parts. Examples of each type of mixture are given.