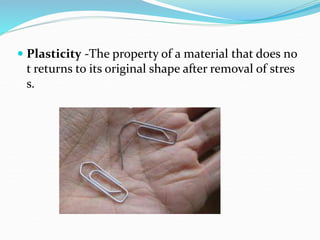
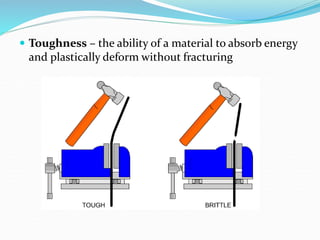
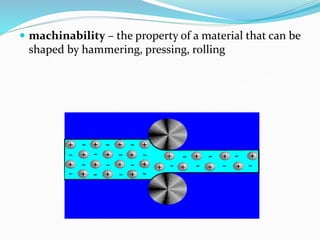
This document discusses the physical, mechanical, and chemical properties of materials. It describes key physical properties like density, specific heat, thermal conductivity, and electrical conductivity. It also outlines important mechanical properties such as tensile strength, ductility, malleability, brittleness, elasticity, plasticity, toughness, and hardness. Finally, it briefly touches on relevant chemical properties including corrosion resistance and erosion resistance.