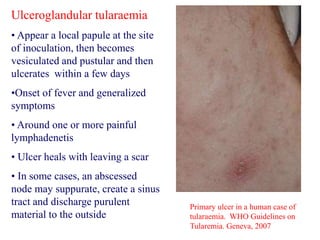
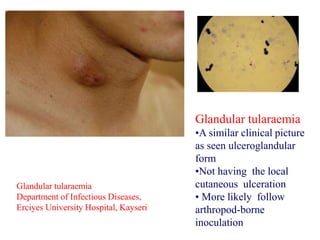
This document provides an overview of tularaemia, a bacterial zoonotic disease caused by Francisella tularensis. It discusses the definition, etiology, epidemiology, transmission, clinical presentation, diagnosis, treatment and prevention. F. tularensis is a highly virulent intracellular pathogen that can cause epidemics in humans and animals. Clinical forms include ulceroglandular, glandular, oculoglandular, oropharyngeal, respiratory, and typhoidal. Diagnosis involves clinical history, microscopy, culture, PCR and serology. Treatment consists of gentamicin or streptomycin for 10 days. Prevention focuses on avoiding contact with infected animals/vectors and disinfecting