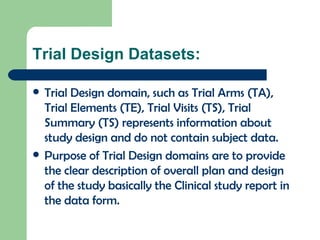
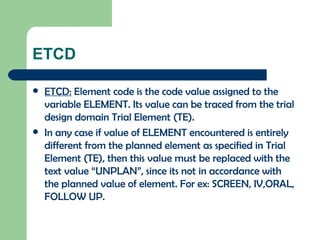
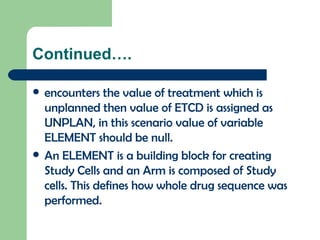
The document discusses several Trial Design domains from CDISC, including Trial Arms (TA), Trial Elements (TE), and Trial Visits (TS). It describes the key variables in each domain like ARMCD, ETCD, ELEMENT, EPOCH, VISITNUM, and start/end rules for trial elements and visits. The domains are used to represent the overall study design and plan without subject-level data.