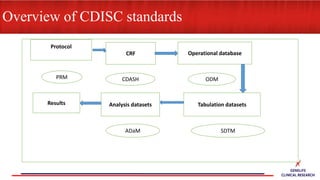
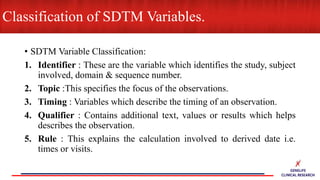
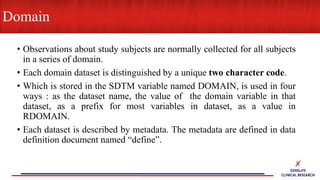
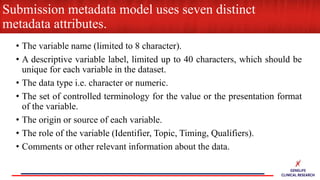
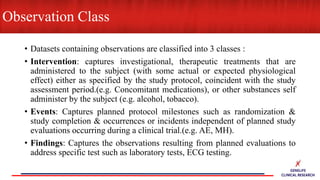
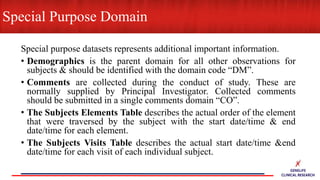
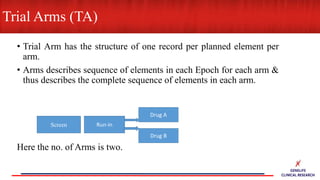
This document provides an overview of CDISC (Clinical Data Interchange Standards Consortium) and SDTM (Standard Data Tabulation Model). It defines these standards, their purpose in establishing common data formats for clinical research, and key concepts in SDTM like domains, variables, qualifiers and time standards. The document also provides examples of how SDTM organizes data from a clinical trial, including adverse events, trial design, and standards for related records.