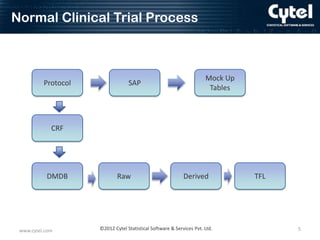
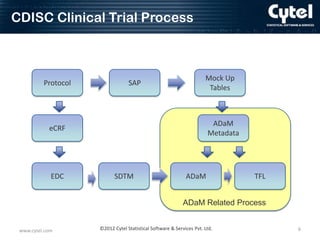
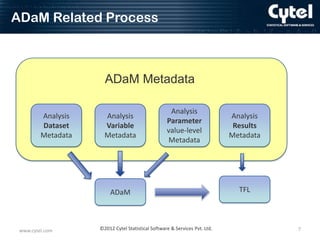
This document discusses ADaM metadata, which provides information about Analysis Data Model (ADaM) datasets. It presents examples of metadata for time to event analyses, including analysis dataset, variable, parameter value-level, and results metadata. The goal of metadata is to specify how to create ADaM and TFL datasets, define information for sponsors, and promote consistency between sites. Benefits include reduced communication needs, easier estimation and planning, and help for inexperienced users. Challenges include initial time/resource investment and determining who maintains the metadata.



![[CB1]Note
to ADaM team: We have elected to use this format for the program names. According to the Study Data Specifications, since the programs created by S
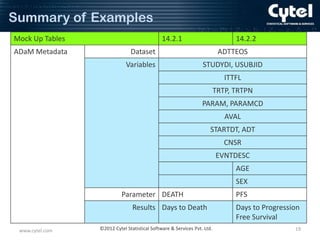
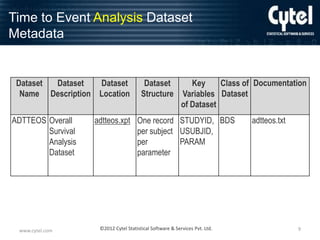
Example 1 : Time to Event Analysis
Variable Metadata including Analysis
Parameter Value-Level Metadata (1)
Dataset Parameter Variable Variable Label Variable Display
Name Identifier Name
Type
Format
Codelist /
Controlled
Terms
Source / Derivation
ADTTEOS *ALL*
STUDYID Study Identifier
text
$20
ADSL.STUDYID
ADTTEOS *ALL*
USUBJID Unique Subject
Identifier
text
$20
ADSL.USUBJID
ADTTEOS *ALL*
ITTFL
Intent-To-Treat
Population Flag
text
$1
Y, null
ADSL.ITTFL
ADTTEOS *ALL*
TRTP
Planned
Treatment
text
$40
Drug 1, Drug 2
ADSL.TRT01P
ADTTEOS *ALL*
TRTPN
Planned
Treatment (N)
integer
1.0
1 = Drug 1,
2 = Drug 2
ADSL.TRT01PN
ADTTEOS PARAMCD
PARAM
Parameter
text
$50
Days to Death
ADTTEOS *ALL*
PARAMCD Parameter Code text
$8
DEATH
www.cytel.com
©2012 Cytel Statistical Software & Services Pvt. Ltd.
10](https://image.slidesharecdn.com/metadataandadam-131126224913-phpapp01/85/Metadata-and-ADaM-10-320.jpg)
![[CB1]Note
to ADaM team: We have elected to use this format for the program names. According to the Study Data Specifications, since the programs created by S
Example 1 : Time to Event Analysis
Variable Metadata including Analysis
Parameter Value-Level Metadata (2)
Dataset Parameter Variable
Name Identifier
Name
Variable
Label
Variable Display
Type Format
Codelist /
Controlled Terms
Source / Derivation
ADTTEOS *ALL*
AVAL
Analysis Value float
8.2
ADT – STARTDT + 1
ADTTEOS *ALL*
STARTDT
YYYYMM
DD10.
ADSL.RANDDT
ADTTEOS *ALL*
ADT
Time to Event integer
Origin Date for
Subject
Analysis Date integer
YYYYMM
DD10.
SAS Date of DS.DSDTC
ADTTEOS *ALL*
CNSR
Censor
integer
1.0
ADTTEOS *ALL*
EVNTDESC Event or
Censoring
Description
text
$40
www.cytel.com
0 for DS.DSDECOD =
„DEATH‟, 1 for any other
study completion
DEATH, COMPLETED DS.DSDECOD
THE STUDY, LOST TO
FOLLOW-UP, AE, PD
0, 1
©2012 Cytel Statistical Software & Services Pvt. Ltd.
11](https://image.slidesharecdn.com/metadataandadam-131126224913-phpapp01/85/Metadata-and-ADaM-11-320.jpg)
![[CB1]Note
to ADaM team: We have elected to use this format for the program names. According to the Study Data Specifications, since the programs created by S
Example 1 : Time to Event ADaM Dataset
USUBJID
TRTP
PARAM
AVAL
STARTDT
001-01-001 Study Drug 1
Days to
Death
157
001-01-002 Study Drug 2
Days to
Death
001-01-003 Study Drug 2
001-01-004 Study Drug 1
www.cytel.com
ADT
CNSR
EVNTDESC
2011-01-04 2011-06-10
1
COMPLETED
THE STUDY
116
2011-02-01 2011-05-28
1
AE
Days to
Death
88
2011-02-05 2011-05-04
0
DEATH
Days to
Death
102
2011-03-20 2011-06-30
1
PD
©2012 Cytel Statistical Software & Services Pvt. Ltd.
12](https://image.slidesharecdn.com/metadataandadam-131126224913-phpapp01/85/Metadata-and-ADaM-12-320.jpg)


![[CB1]Note
to ADaM team: We have elected to use this format for the program names. According to the Study Data Specifications, since the programs created by S
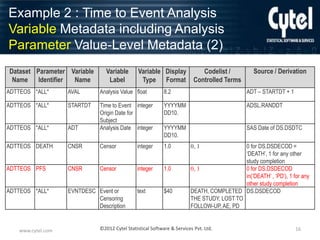
Example 2 : Time to Event Analysis
Variable Metadata including Analysis
Parameter Value-Level Metadata (1)
Dataset Parameter Variable Variable Label Variable Display
Name Identifier Name
Type
Format
Source / Derivation
Codelist /
Controlled Terms
ADTTEOS *ALL*
STUDYID Study Identifier
text
$20
ADSL.STUDYID
ADTTEOS *ALL*
USUBJID Unique Subject
Identifier
text
$20
ADSL.USUBJID
ADTTEOS *ALL*
ADTTEOS *ALL*
ADTTEOS *ALL*
AGE
SEX
ITTFL
Age
Sex
Intent-To-Treat
Population Flag
integer
text
text
3.0
$1
$1
Y, null
ADSL.AGE
ADSL.SEX
ADSL.ITTFL
ADTTEOS *ALL*
TRTP
Planned
Treatment
text
$40
Drug 1, Drug 2
ADSL.TRT01P
ADTTEOS *ALL*
TRTPN
Planned
Treatment (N)
integer
1.0
1 = Drug 1,
2 = Drug 2
ADSL.TRT01PN
ADTTEOS PARAMCD
PARAM
Parameter
text
$50
ADTTEOS *ALL*
PARAMCD Parameter Code text
Days to Death
Days to Progression
Free Survival
DEATH
PFS
www.cytel.com
$8
©2012 Cytel Statistical Software & Services Pvt. Ltd.
15](https://image.slidesharecdn.com/metadataandadam-131126224913-phpapp01/85/Metadata-and-ADaM-15-320.jpg)
![[CB1]Note
to ADaM team: We have elected to use this format for the program names. According to the Study Data Specifications, since the programs created by S
Example 2 : Time to Event ADaM Dataset
USUBJ AGE SEX TRTP
ID
PARAM
AVA STAR
L
TDT
ADT
CNSR
EVNTDESC
001-01001
43
M
Study
Drug 1
Days to Death
157
201101-04
201106-10
1
COMPLETED
THE STUDY
001-01002
57
F
Study
Drug 2
Days to Death
116
201102-01
201105-28
1
AE
001-01003
71
M
Study
Drug 2
Days to Death
88
201102-05
201105-04
0
DEATH
001-01004
55
F
Study
Drug 1
Days to Death
102
201103-20
201106-30
1
PD
001-01001
43
M
Study
Drug 1
Days to Progression 157
Free Survival
201101-04
201106-10
1
COMPLETED
THE STUDY
001-01002
57
F
Study
Drug 2
Days to Progression 116
Free Survival
201102-01
201105-28
1
AE
001-01003
71
M
Study
Drug 2
Days to Progression 88
Free Survival
201102-05
201105-04
0
DEATH
001-01004
55
F
Study
Drug 1
Days to Progression 102
Free Survival
201103-20
201106-30
0
PD
www.cytel.com
©2012 Cytel Statistical Software & Services Pvt. Ltd.
17](https://image.slidesharecdn.com/metadataandadam-131126224913-phpapp01/85/Metadata-and-ADaM-17-320.jpg)