This document discusses how predictive analytics can help enable personalized health care through three main points:
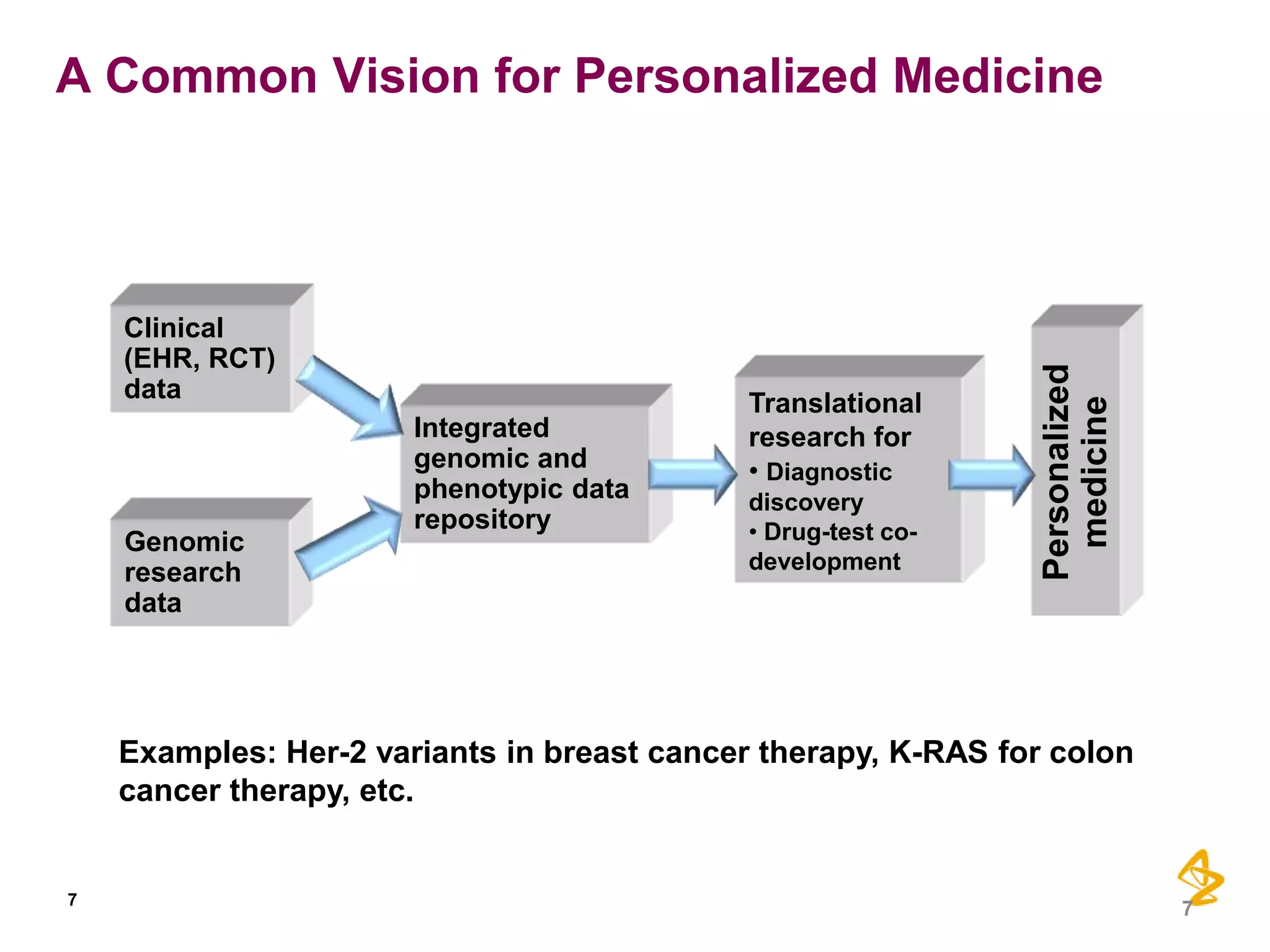
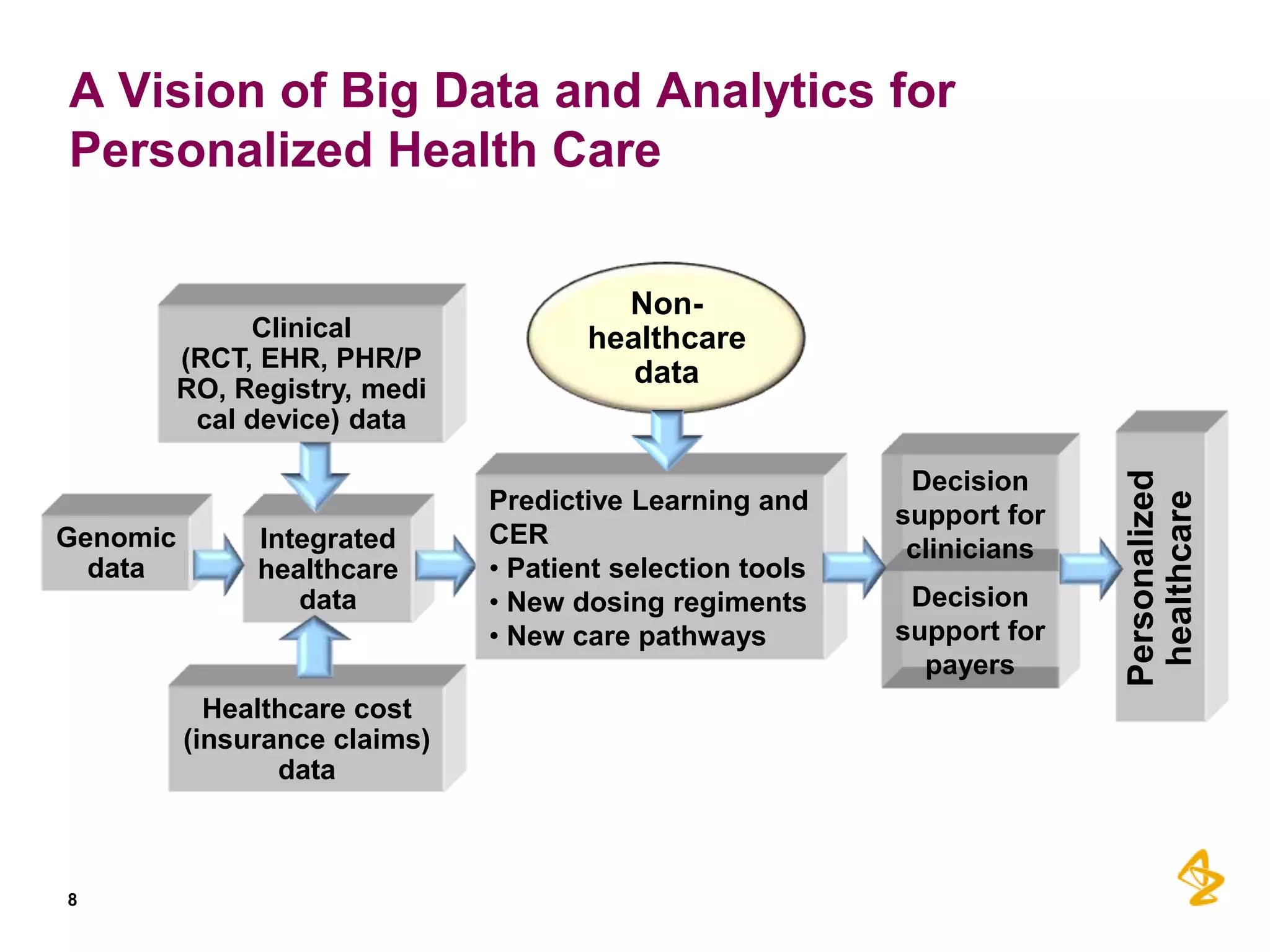
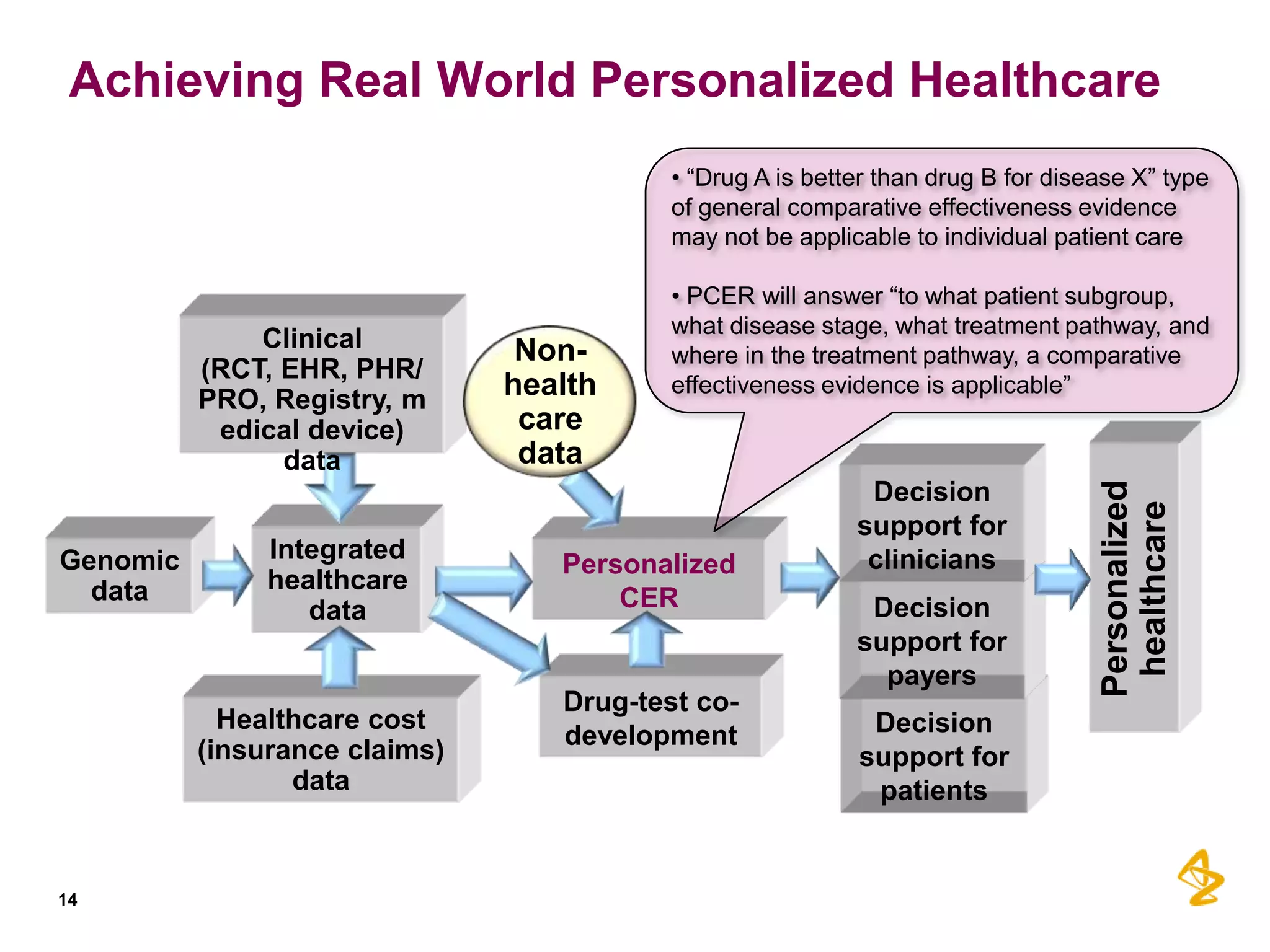
1) Integrating diverse data sources like genomics, healthcare records, and insurance claims can provide insights for personalized care, drug development, and comparative effectiveness research.
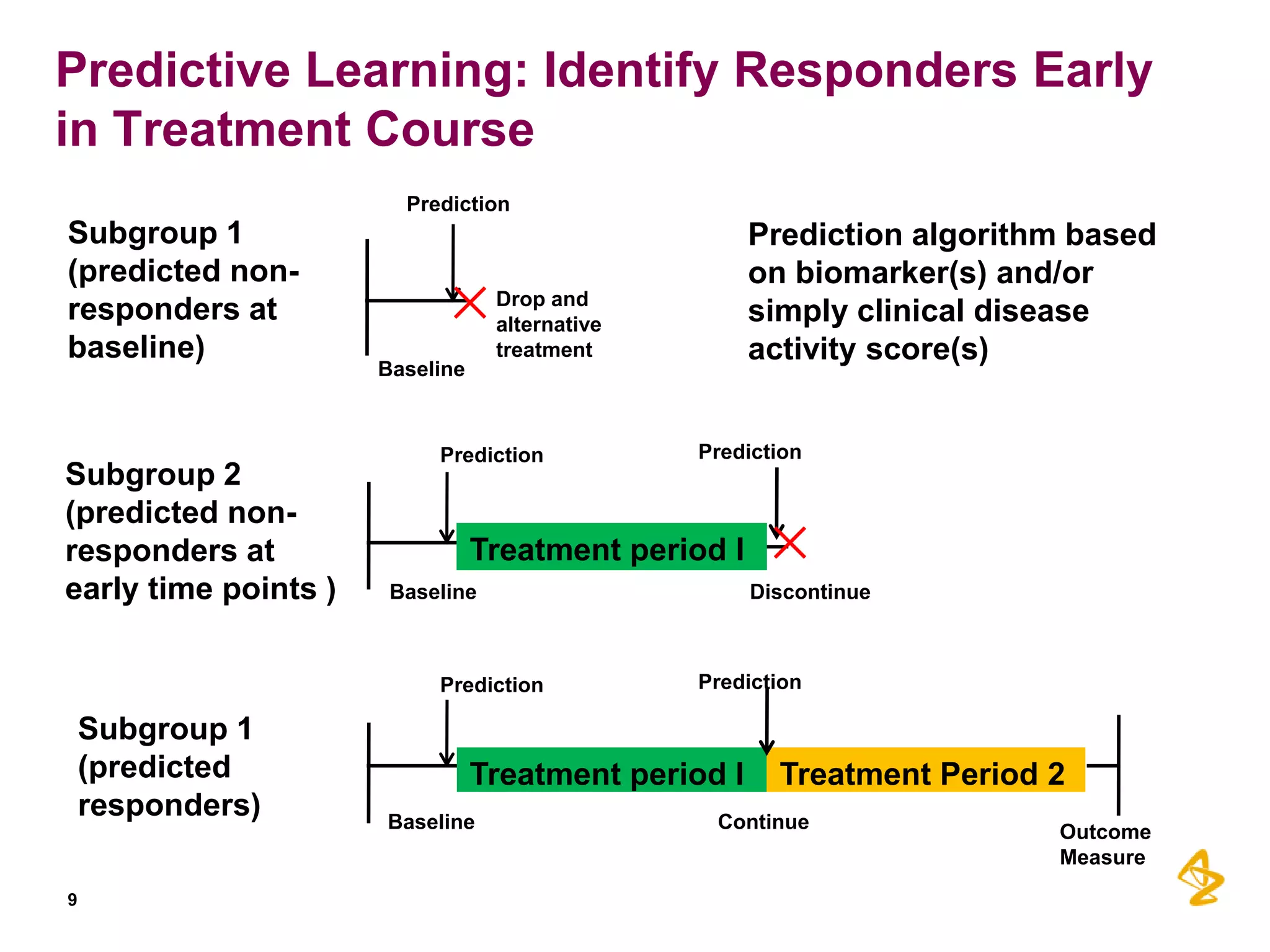
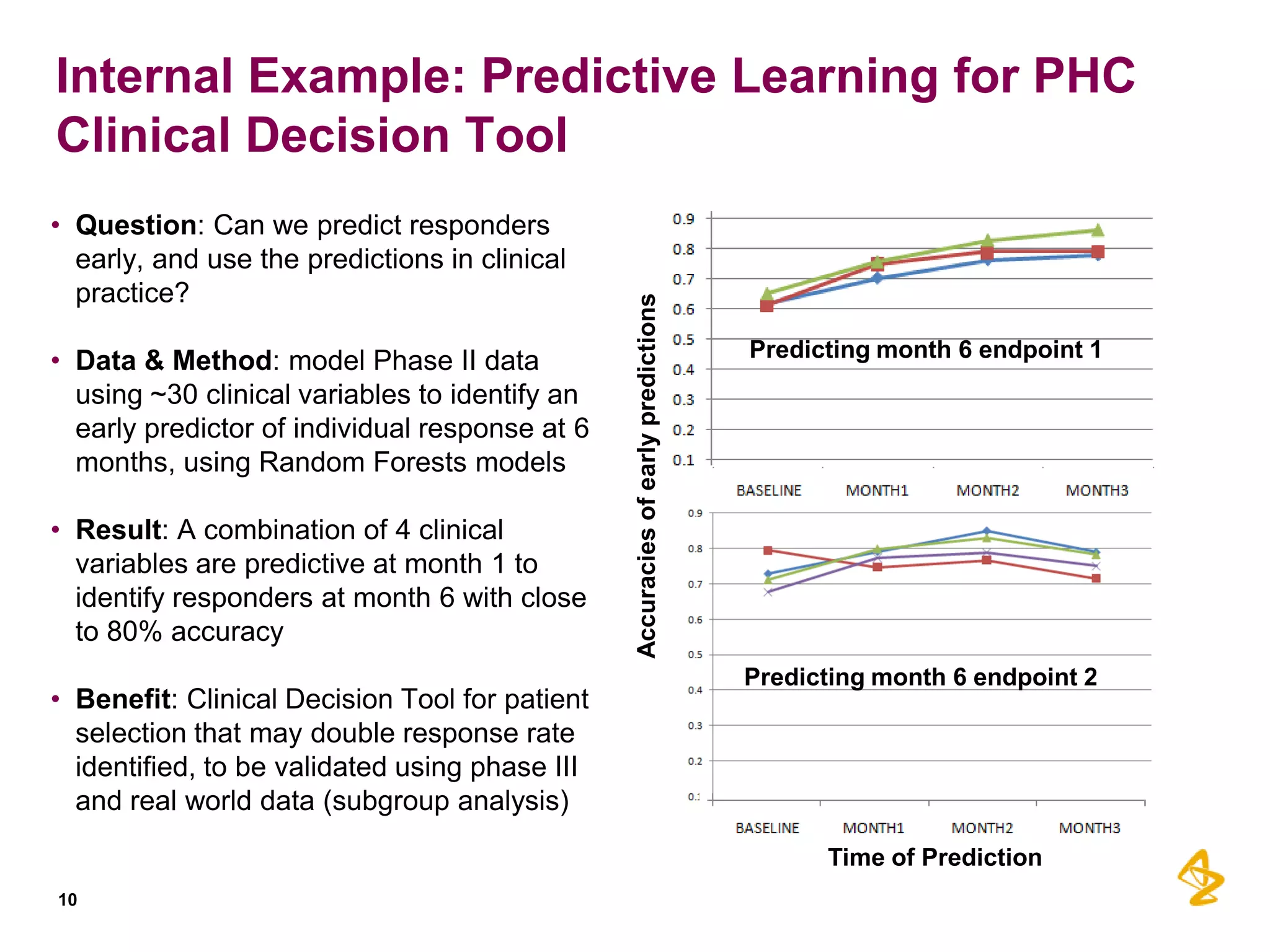
2) Predictive models built using data from clinical trials can identify subgroups of patients most likely to respond or not respond to treatments early in the treatment course, improving outcomes.
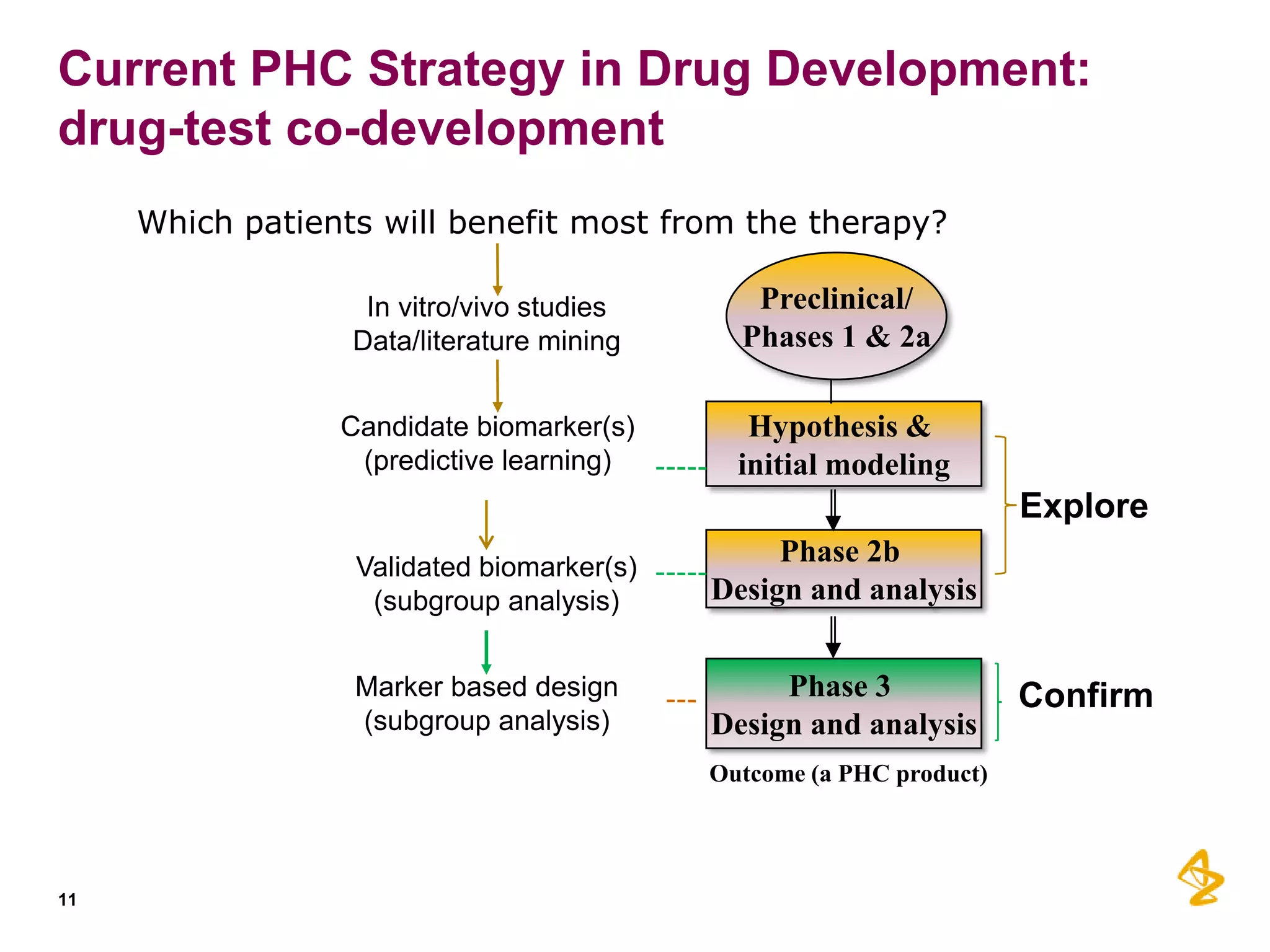
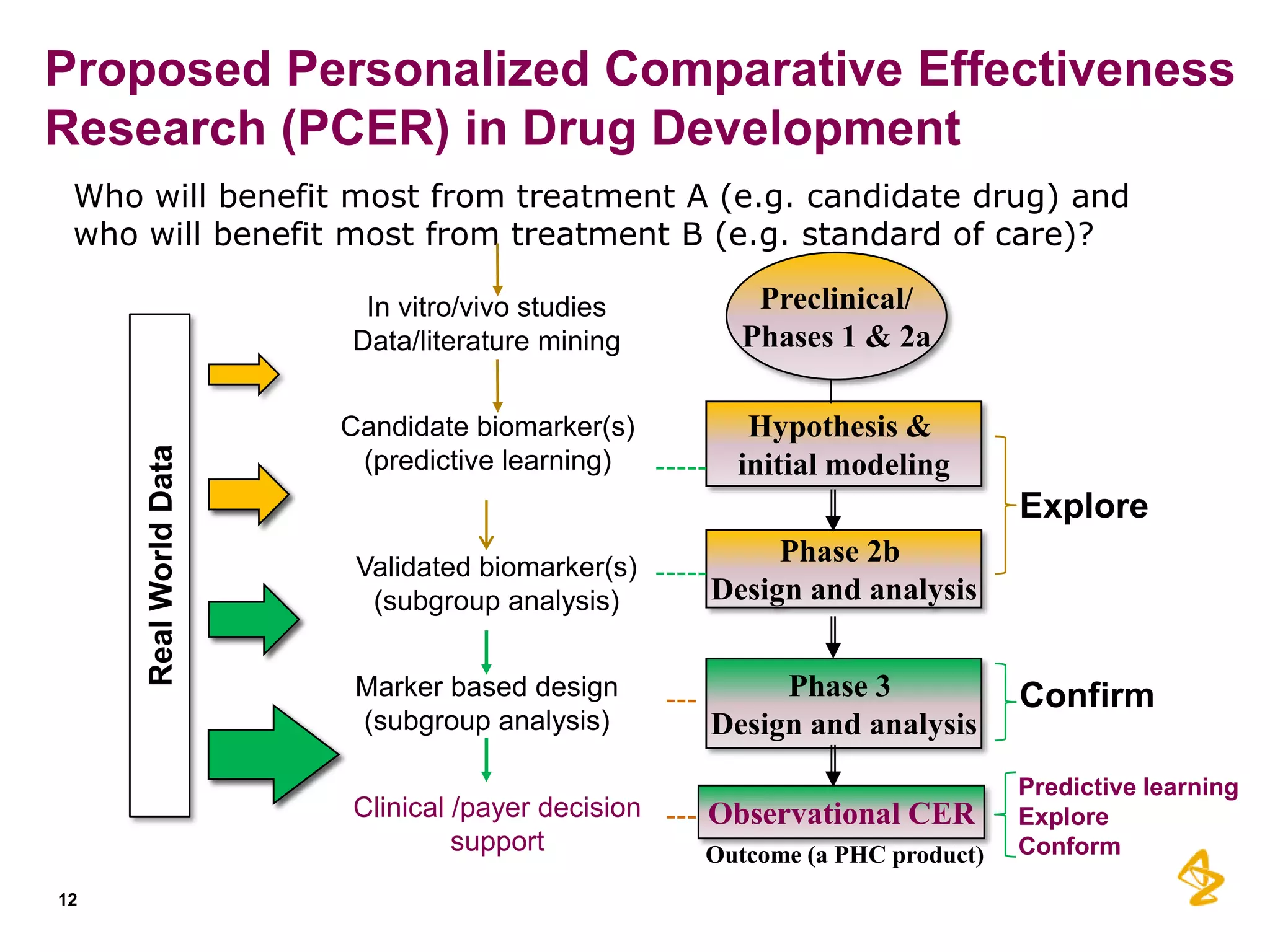
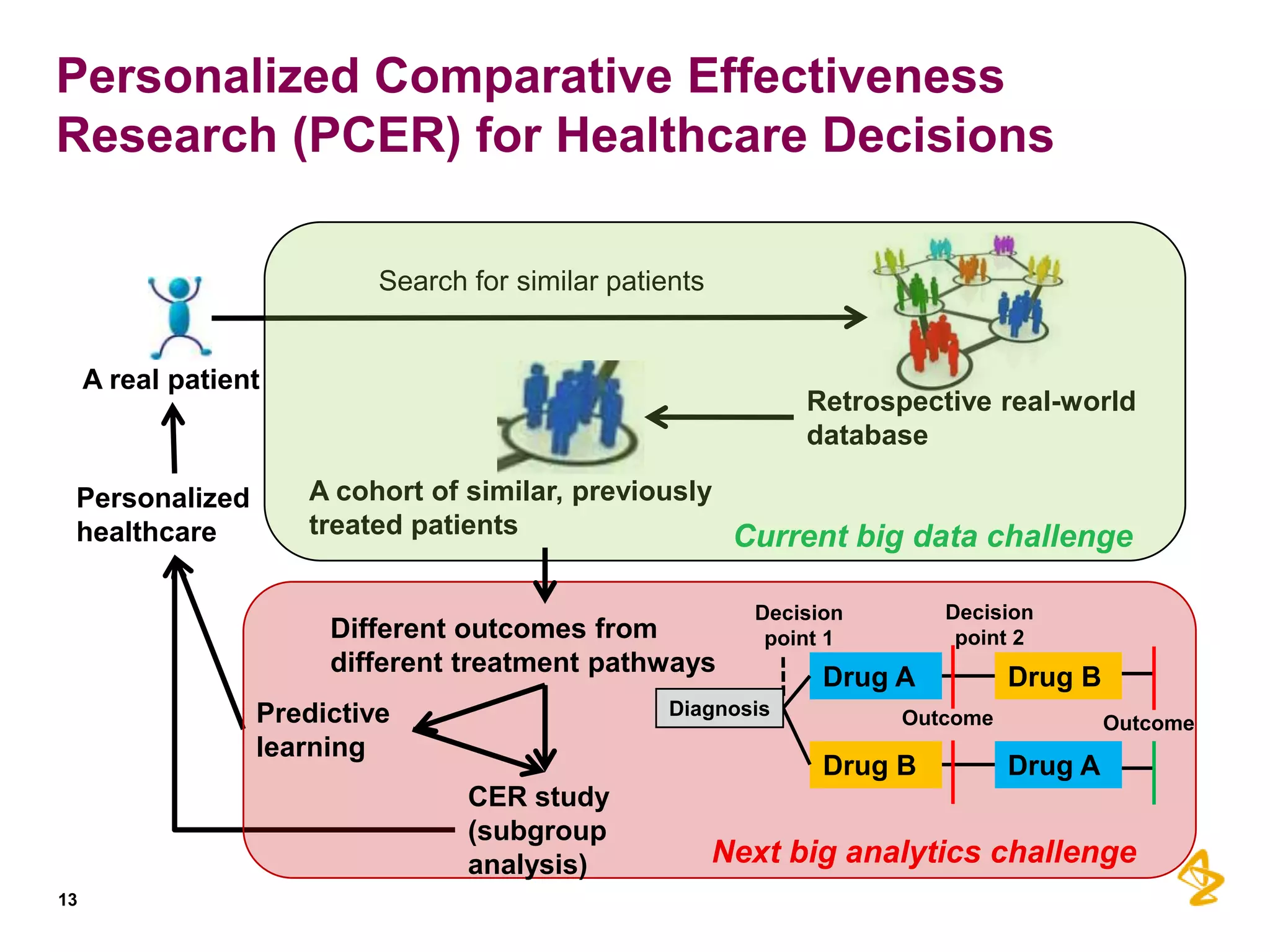
3) Personalized comparative effectiveness research aims to determine which treatments work best for which patient subgroups and disease stages by integrating real-world data and predictive analytics into drug development and clinical decision-making.