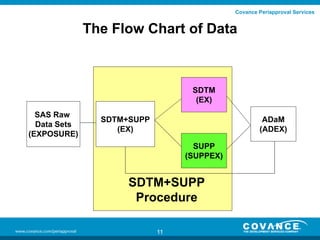
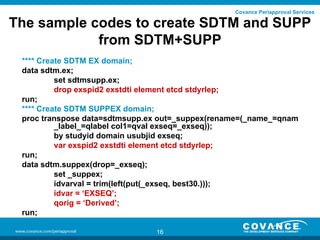
This document discusses how to validate the SDTM SUPPQUAL dataset. It recommends creating separate SUPP datasets for each SDTM domain to make validation easier. It also recommends creating SDTM+SUPP datasets that combine each SDTM domain with its related supplemental variables for improved traceability. The document provides steps for validating SDTM+SUPP datasets and using a define document and macro to split them into separate SDTM and SUPP datasets during the validation process.