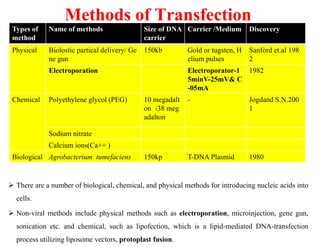
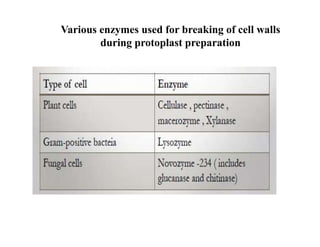
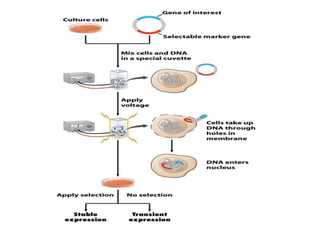
The document discusses transfection techniques for introducing nucleic acids into eukaryotic cells, focusing on methods such as electroporation and protoplast fusion. These methods allow for gene transfer between species, improving microbial strains for industrial applications. Specific techniques and their advantages, including spontaneous and induced fusion processes, are outlined, as well as their applications in genetic enhancement and production improvements.