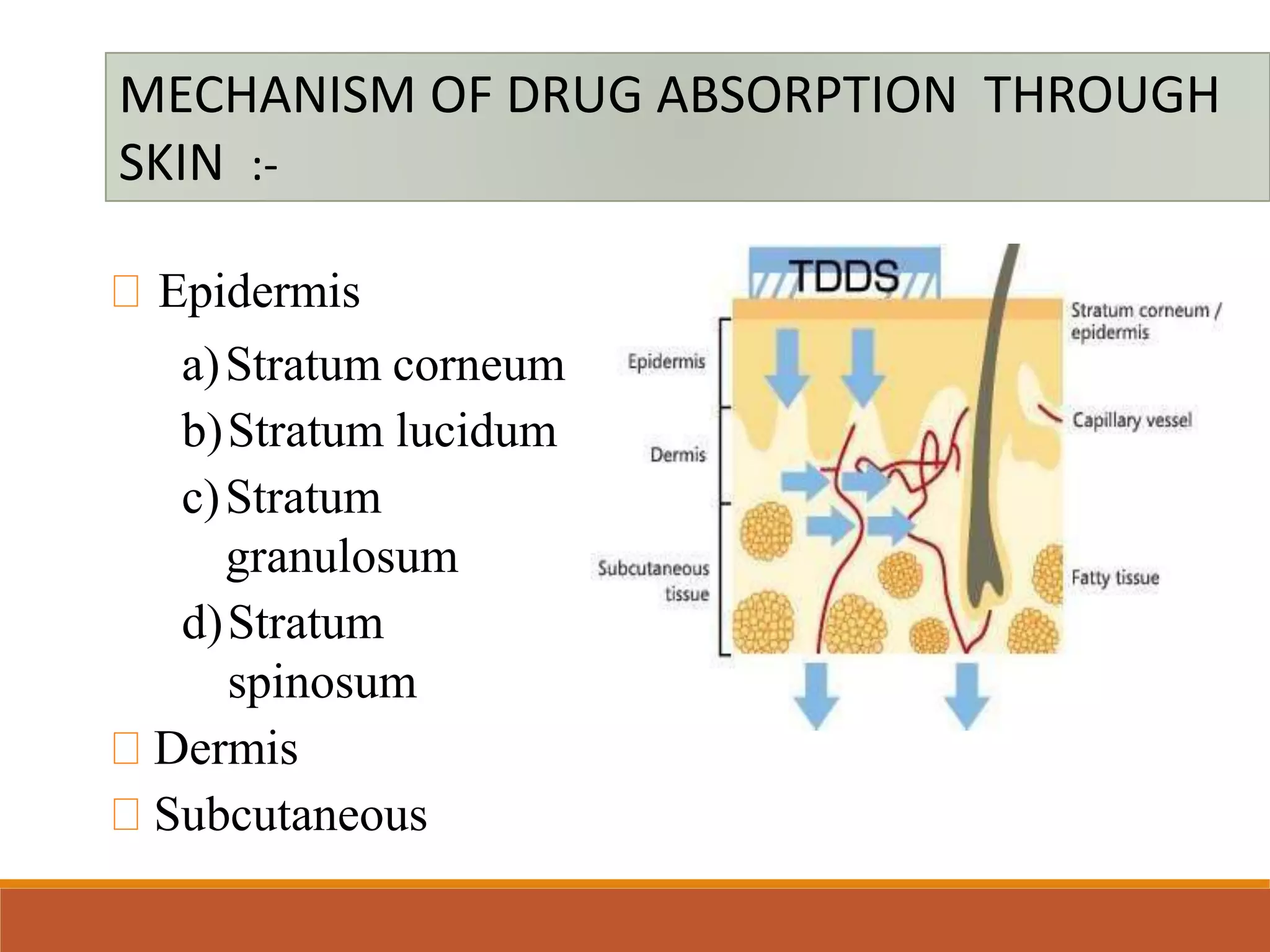
This document provides an overview of transdermal drug delivery systems (TDDS). It defines TDDS and discusses the mechanism of drug absorption through skin, including the transfollicular, transcellular and intercellular routes. It outlines the factors affecting transdermal permeability such as lipid solubility, partition coefficient and pH condition. It also describes types of TDDS, permeation enhancers, recent techniques to enhance delivery, advantages and disadvantages of TDDS, evaluation parameters and concludes that TDDS technologies are becoming widely used in the pharmaceutical industry.