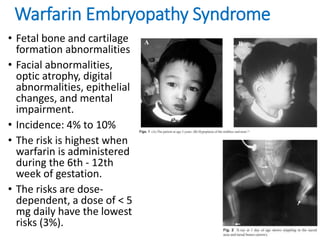
This document discusses anticoagulation options during pregnancy for conditions requiring it, such as mechanical heart valves or blood clotting disorders. It describes that warfarin, unfractionated heparin, and low-molecular weight heparin are the three most common agents considered. While warfarin is very effective, it can harm the fetus if used in early pregnancy. Unfractionated heparin and low-molecular weight heparin do not cross the placenta and are safer options for early pregnancy, with low-molecular weight heparin requiring anti-Xa level monitoring. The document provides guidelines for using warfarin or heparins based on the trimester and any needed dose






![CLASS I
• Therapeutic anticoagulation with frequent monitoring is
recommended for all pregnant patients with a
mechanical prosthesis. (Level of Evidence: B)
• Warfarin is recommended in pregnant patients with a
mechanical prosthesis to achieve a therapeutic INR in the
second and third trimesters. (Level of Evidence: B)
• Discontinuation of warfarin with initiation of intravenous
UFH (with an activated partial thromboplastin time [aPTT] >2 times
control) is recommended before planned vaginal delivery
in pregnant patients with a mechanical prosthesis. (Level
of Evidence: C)
• Low-dose aspirin (75mg to 100mg) once per day is
recommended for pregnant patients in the second and
third trimesters with either a mechanical prosthesis or
bioprosthesis. (Level of Evidence: C)](https://image.slidesharecdn.com/antithromboticinpregnancy-170604075935/85/Antithrombotic-in-pregnancy-10-320.jpg)



