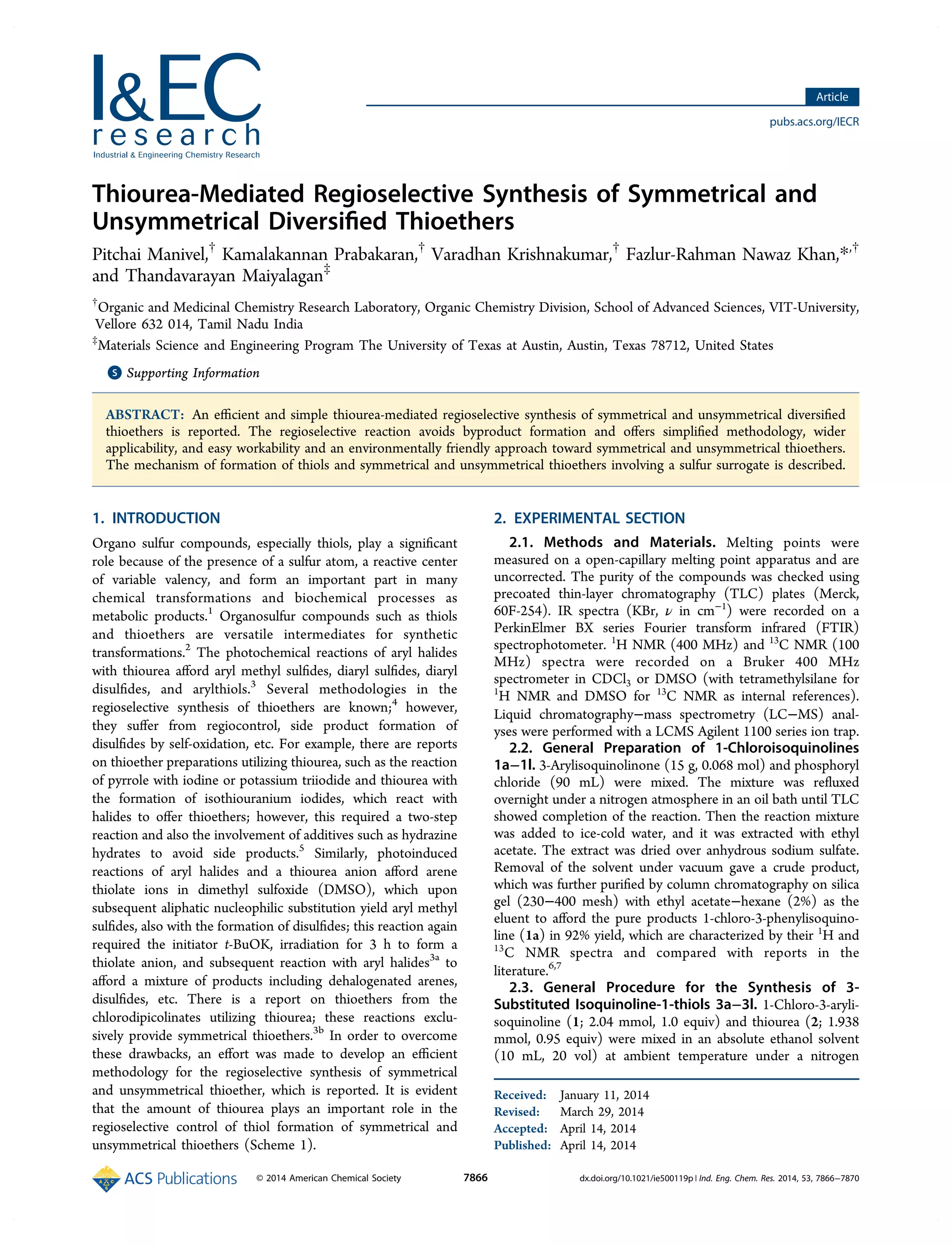
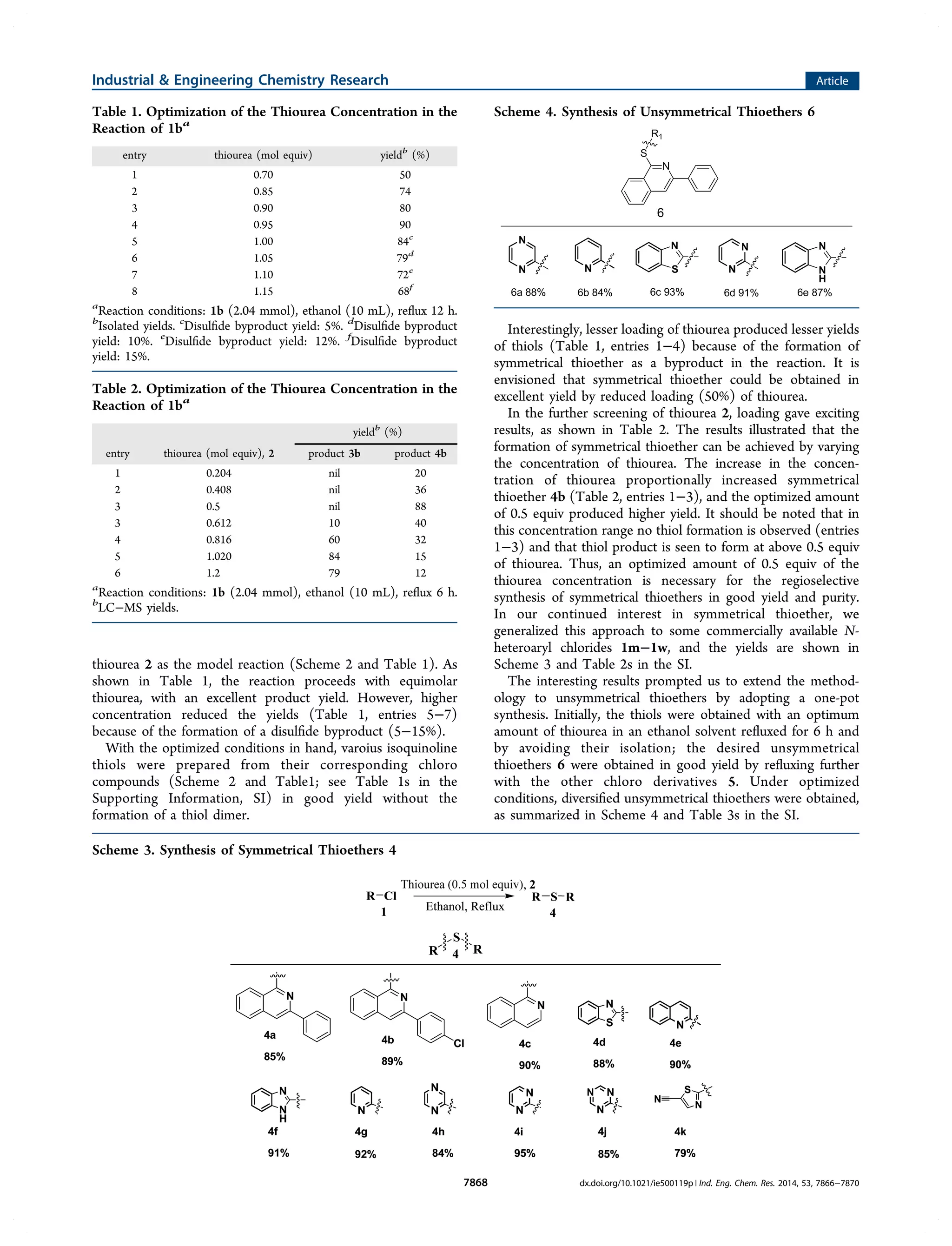
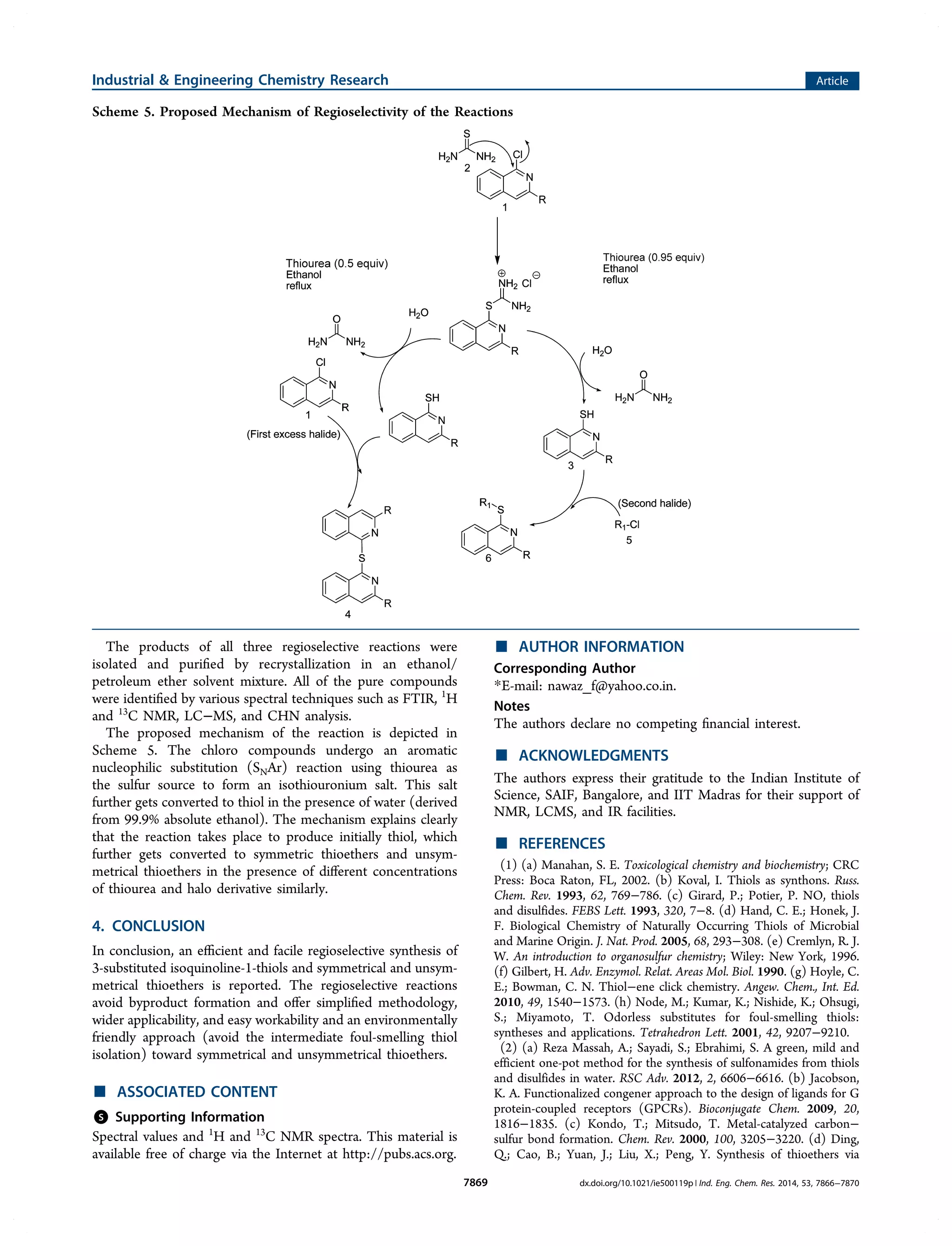
This document describes a method for the regioselective synthesis of symmetrical and unsymmetrical thioethers from chloroisoquinolines using thiourea. The reaction produces isoquinoline-1-thiols under optimized thiourea conditions, and varying the amount of thiourea allows for the selective formation of either symmetrical thioethers or unsymmetrical thioethers. A proposed mechanism indicates that thiourea acts as a sulfur source in an aromatic nucleophilic substitution reaction to form an isothiouronium salt intermediate, which then produces the thiol or thioether products. This method avoids byproduct formation and provides a simplified and environmentally friendly approach for synthesizing various organosulfur

![metal-free reductive coupling of tosylhydrazones with thiols. Org.
Biomol. Chem. 2011, 9, 748−751. (e) Badoiu, A.; Bernardinelli, G.;
Besnard, C.; Kundig, E. P. Asymmetric ruthenium-catalyzed 1,4-
additions of aryl thiols to enones. Org. Biomol. Chem. 2010, 8, 193−
200. (f) Levin, E.; Anaby, A.; Diesendruck, C. E.; Berkovich-Berger,
D.; Fuchs, B.; Lemcoff, N. G. Oligomerisation reactions of beta
substituted thiols in water. RSC Adv. 2013.
(3) (a) Argüello, J. E.; Schmidt, L. C.; Peñéñory, A. B. “One-Pot”
Two-Step Synthesis of Aryl Sulfur Compounds by Photoinduced
Reactions of Thiourea Anion with Aryl Halides. Org. Lett. 2003, 5,
4133−4136. (b) Markees, D. G. Derivatives of 4-Mercaptodipicolinic
Acid. J. Org. Chem. 1963, 28, 2530−2533.
(4) (a) Kondrashov, E.; Rudyakova, E.; Rozentsveig, I.; Ushakova, I.;
Rozentsveig, G.; Savosik, V.; Chernyshev, K.; Krivdin, L.; Levkovskaya,
G. Reactions of N-(Polychloroethylidene) arene and trifluorometha-
nesulfonamides with indoles. Russ. J. Org. Chem. 2008, 44, 86−94.
(b) Salvatore, R. N.; Smith, R. A.; Nischwitz, A. K.; Gavin, T. A mild
and highly convenient chemoselective alkylation of thiols using
Cs2CO3−TBAI. Tetrahedron Lett. 2005, 46, 8931−8935. (c) Sudalai,
A.; Kanagasabapathy, S.; Benicewicz, B. C. Phosphorus Pentasulfide: A
Mild and Versatile Catalyst/Reagent for the Preparation of
Dithiocarboxylic Esters. Org. Lett. 2000, 2, 3213−3216. (d) Kanagasa-
bapathy, S.; Sudalai, A.; Benicewicz, B. C. Montmorillonite K 10-
catalyzed regioselective addition of thiols and thiobenzoic acids onto
olefins: an efficient synthesis of dithiocarboxylic esters. Tetrahedron
Lett. 2001, 42, 3791−3794. (e) Thang, S. H.; Chong, Y. K.;
Mayadunne, R. T. A.; Moad, G.; Rizzardo, E. A novel synthesis of
functional dithioesters, dithiocarbamates, xanthates and trithiocarbon-
ates. Tetrahedron Lett. 1999, 40, 2435−2438. (f) Movassagha, B.;
Shaygana, P. Michael addition of thiols to α,β-unsaturated carbonyl
compounds under solvent-free conditions. ARKIVOC 2006, 12, 130−
137. (g) Falck, J. R.; Lai, J.-Y.; Cho, S.-D.; Yu, J. Alkylthioether
synthesis via imidazole mediated mitsunobu condensation. Tetrahedron
Lett. 1999, 40, 2903−2906. (h) Perrier, S.; Takolpuckdee, P.
Macromolecular design via reversible addition−fragmentation chain
transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci.,
Part A: Polym. Chem. 2005, 43, 5347−5393.
(5) Mirskova, A.; Levkovskaya, G.; Mirskov, R.; Voronkov, M.
Hydroxyalkylammonium salts of organylsulfanyl (sulfonyl) acetic
acidsNew stimulators of biological processes. Russ. J. Org. Chem.
2008, 44, 1478−1485.
(6) (a) Krishnakumar, V.; Kumar, K. M.; Mandal, B. K.; Khan, F. R.
N. Zinc Oxide Nanoparticles Catalyzed Condensation Reaction of
Isocoumarins and 1,7-Heptadiamine in the Formation of Bis-
Isoquinolinones. Sci. World J. 2012, 2012. (b) Nawaz Khan, F.;
Manivel, P.; Prabakaran, K.; Jin, J. S.; Jeong, E. D.; Kim, H. G.;
Maiyalagan, T. Iron-oxide nanoparticles mediated cyclization of 3-(4-
chlorophenyl)-1-hydrazinylisoquinoline to 1-(4,5-dihydropyrazol-1-yl)
isoquinolines. Res. Chem. Intermed. 2012, 38, 571−582. (c) Prabakaran,
K.; Nawaz Khan, F.; Jin, J. S. Ligand-free, PdCl2(PPh3)2 catalyzed,
microwave-assisted Suzuki coupling of 1-chloro-3-phenylisoquinoline
in the synthesis of diversified 1,3-disubstituted isoquinolines. Res.
Chem. Intermed. 2012, 38, 337−346. (d) Prabakaran, K.; Nawaz Khan,
F.; Jin, J. S.; Manivel, P. Indium bromide catalysed, ultrasound-assisted,
regio-selective synthesis of ethyl-5-(trifluoromethyl)-1-(3-substituted
isoquinolin-1-yl)-1H-pyrazole-4-carboxylates. Res. Chem. Intermed.
2012, 38, 429−441. (e) Khan, F. N.; Manivel, P.; Krishnakumar, V.;
Hathwar, V. R.; Ng, S. W. 1-[3-(4-Chlorophenyl)isoquinolin-1-yl]-3,5-
diphenyl-1H-pyrazole. Acta Crystallogr., Sect. E: Struct. Rep. Online
2010, 66 (2), o369. (f) Manivel, P.; Khan, F. N. Synthesis of some new
2,4-disubstituted hydrazinothiazoles and 2,5-disubstituted thiazolidi-
nones. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 184, 2910−2922.
(g) Manivel, P.; Khan, F. N.; Hatwar, V. R Synthesis of diversified
thioethers, 1- aroylalkylisoquinolin-1-yl thioethers, by electrophilic s-
alkylation of 3-phenylisoquinoline-1(2H)-thione. Phosphorus, Sulfur
Silicon Relat. Elem. 2010, 185, 1932−1942.
(7) (a) Prabakaran, K.; Manivel, P.; Nawaz Khan, F. An effective
BINAP and microwave accelerated palladium-catalyzed amination of
1-chloroisoquinolines in the synthesis of new 1,3-disubstituted
isoquinolines. Tetrahedron Lett. 2010, 51, 4340−4343. (b) Prabakaran,
K.; Nawaz Khan, F.; Jin, J. S. An efficient copper-free Pd(OAc)2/
Ruphos-catalyzed Sonogashira coupling of 1-chloroisoquinolines in the
formation of 1-alkynyl-3-substituted isoquinolines. Tetrahedron Lett.
2011, 52, 2566−2570.
Industrial & Engineering Chemistry Research Article
dx.doi.org/10.1021/ie500119p | Ind. Eng. Chem. Res. 2014, 53, 7866−78707870](https://image.slidesharecdn.com/thiourea-mediatedregioselectivesynthesisofsymmetricalandunsymmetricaldiversifiedthioetheres-140619220358-phpapp01/75/Thiourea-mediated-regioselective-synthesis-of-symmetrical-and-unsymmetrical-diversified-thioetheres-5-2048.jpg)