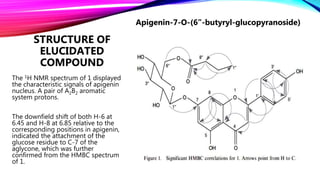
1. A new acylated apigenin glucoside compound was isolated from the leaves of Phyllanthus emblica and its structure was elucidated.
2. Spectroscopic analysis including 1D and 2D NMR, UV, and ESI-MS revealed the compound's structure as apigenin-7-O-(6”-butyryl--glucopyranoside).
3. The compound exhibited potent cytotoxicity against tumor cell lines based on SRB assays, indicating potential anticancer properties. Four other compounds were also isolated and identified from the plant.








![ISOLATION AND
STRUCTURE ELUCIDATION
Several reports about this plant have shown that the fruits are rich in,
Vitamin C (Aescorbic Acid) [1], Mucic Acid and Tannins [2,3], Flavanone Glycosides [4], Phenolic
glycosides [5], Sesquiterpenoids [6], Norsesquiterpenoids [7], Phenolic Acids [8] and Flavonol
Glycosides [9].
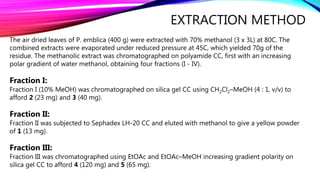
In this report the isolation and structure elucidation of a new acylated flavone glucoside from the leaves of
P. emblica,
Isolated compounds From leaves Phyllanthus Emlica:
1) apigenin-7-O-(6”-butyryl--glucopyranoside) (1)
2) gallic acid (2),
3) methyl gallate (3),
4) 1,2,3,4,6-penta-O-galloylglucose (4),
5) luteolin-40-O-neohesperidoside (5). (rare flavone glycoside)
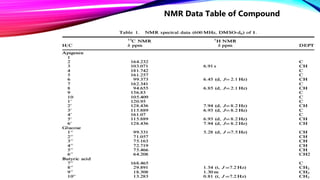
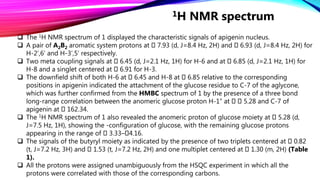
The structure elucidation of these compounds was established on the basis of 1D and 2D NMR experiments.
Among the isolated compounds, (1) exhibited a potent cytotoxicity against cultured tumor cell lines in vitro
using the SRB (sulforhodamine-B) method for cytotoxicity evaluation.](https://image.slidesharecdn.com/researchjournal-170604010703/85/Research-journal-9-320.jpg)


![SUMMARY OF
METHODS
The ESI-MS (Electrospray Ionization Mass Spectrometry) spectrum (negative mode- ion) of 1
depicted the [M-1]+peak at 501 m/z, confirming the molecular formula C25H26O11 of compound 1.
Mass Spectrum
From these results, compound 1 was identified as:
apigenin-7-O-(600-butyryl--glucopyranoside
The rest of the compounds were identified by comparing their spectral data (1H NMR,13C NMR
and ESI-MS) to those published, gallic acid, methyl gallate, 1,2,3,4,6-penta galloylglucose
and luteolin-4’- neohespridoside.
Summary
References:
Electrospray and MALDI Mass Spectrometry Fundamentals, Instrumentation, Practicalities, and
Biological Applications Second Edition Edited by Richard B. Cole (WILEY)
Indian Medicinal Plants An Illustrated Dictionary Author C.P. Khare (Springer)
INTRODUCTION TO SPECTROSCOPY 5th Ed Donald L. Pavia
International Journal Of Ayurvedic And Herbal Medicine 2:5 (2012) 828:834](https://image.slidesharecdn.com/researchjournal-170604010703/85/Research-journal-16-320.jpg)
