Coral-shaped zinc oxide (ZnO) nanostructures were successfully synthesized via a combustion method using glycine as a fuel and zinc nitrate and lanthanum nitrate as precursors. Transmission electron microscopy showed the ZnO to have a coral shape with pore sizes of 10-50 nm and grain size of around 15 nm. X-ray diffraction analysis confirmed the hexagonal wurtzite crystal structure of both pure and lanthanum-doped ZnO. The lattice parameters increased with increasing lanthanum concentration, indicating incorporation of lanthanum ions into the ZnO lattice. Ultraviolet-visible spectroscopy showed the band gap increased and absorbance decreased in the near UV region with higher lanthanum doping
![Optical studies of nano-structured La-doped ZnO prepared by
combustion method
L. Arun Jose a
, J. Mary Linet a
, V. Sivasubramanian b
, Akhilesh K. Arora c
, C. Justin Raj d
,
T. Maiyalagan e
, S. Jerome Das a,n
a
Department of Physics, Loyola College, Chennai 600034, India
b
Light Scattering Studies Section, IGCAR, Kalpakkam 603102, India
c
Condensed Matter Physics Division, IGCAR, Kalpakkam 603102, India
d
Pusan National University, Jangjeon, Geumjeong, Busan 609 735, South Korea
e
School of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore 639 798, Singapore
a r t i c l e i n f o
Article history:
Received 4 August 2011
Received in revised form
13 March 2012
Accepted 14 March 2012
Available online 21 April 2012
Keywords:
Doping
Semiconducting II–VI materials
Nano-structures
combustion
X-ray diffraction spectra
Zinc compounds
Rare earth compounds
a b s t r a c t
Coral-shaped nano-structured zinc oxide (ZnO) was successfully synthesized and La-
doped via a facile combustion process using glycine as a fuel. The auto-ignition
(at $185 1C) of viscous reactants zinc nitrate and glycine resulted in ZnO powders.
Hexagonal wurtzite structure of pure and doped ZnO powder was confirmed by X-ray
powder diffraction analysis. The transmission electron micrograph shows that the
nano-structured ZnO is coral-shaped and possess maximal pore ($10–50 nm pore size)
density in it and the grain size is approximately about 15 nm. Addition of dopants
subsequently alters the structural and optical properties which were confirmed by
UV–VIS studies.
& 2012 Elsevier Ltd. All rights reserved.
1. Introduction
Nano-structured metal oxide semiconductors are gain-
ing attention due to their wide band-gap and related
properties [1]. Recent decades are witnessed with
researchers paying much interest in synthesis and char-
acterization of II–VI group semiconducting materials at
nano- [2] and bulk [3] levels. Zinc oxide (ZnO) is a widely
exploited, due to its excellent physical and chemical
properties. Numerous researchers proposed the solution
combustion method to synthesize simple and mixed
metal oxides [4–9]. Normally ZnO is doped with different
types of metallic ions in order to enhance the optical and
conducting properties [10–14]. The exceptional interest
on ZnO may be seen in the recent literatures. The
modified ZnO may be used as a base material for diluted
magnetic semiconductors [15–18], gas sensors [19],
photocatalysts [20], field-effect transistors [21,22], light-
emitting materials [23–25], solar cells [26,27] and biolo-
gical systems (drug delivery, bio-imaging, etc.) [28,29]. In
the recent times, rare earth metal-doped ZnO (e.g., Tb, Er,
Eu, Dy and Sm) has been broadly researched and concen-
trated on luminescence properties [24,30–33]. Lantha-
num (La)-doped ZnO nano-structures exhibit excellent
photocatalytic activity and gas sensitivity [20,34–36].
Nano-sized ZnO has been synthesized by the solution
combustion method and there are no literature references
for La-doped ZnO using this method. Current work is focused
on investigating the result of La doping concentration on the
Contents lists available at SciVerse ScienceDirect
journal homepage: www.elsevier.com/locate/mssp
Materials Science in Semiconductor Processing
1369-8001/$ - see front matter & 2012 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.mssp.2012.03.011
n
Corresponding author. Tel.: þ91 44 2817 5662;
fax: þ91 44 2817 5566.
E-mail addresses: sjeromedas2004@yahoo.com,
jerome@loyolacollege.edu (S. Jerome Das).
Materials Science in Semiconductor Processing 15 (2012) 308–313](https://image.slidesharecdn.com/opticalstudiesofnano-structuredla-dopedznopreparedbycombustionmethod-160428034631/75/Optical-studies-of-nano-structured-la-doped-zn-o-prepared-by-combustion-method-1-2048.jpg)
![microstructure and optical properties of ZnO nano-structure
prepared by the combustion method.
2. Experimental details
Distinct from usual thermal evaporation, ZnO nano-
structures were prepared by the combustion method, which
allows efficient synthesis of nano-size materials. This pro-
cess involves a self-sustained reaction in homogeneous
solution of different oxidizers (e.g., metal nitrates) and fuels
(e.g., urea, glycine, citric acid, hydrazides). Depending on the
type of precursors, and the suitable conditions for chemical
reaction to take place, zinc nitrate (Zn(NO3)2 Á 6H2O) was
chosen as an oxidizer and glycine (NH2CH2COOH) as a fuel,
since its combustion heat (À3.24 kcal/g) is more negative
when compared with urea (À2.98 kcal/g) or citric acid
(À2.76 kcal/g) [36]. Lanthanum nitrate (La(NO3)2 Á 6H2O) is
added to zinc nitrate with required molar ratio and glycine
is also added along with it, in a molar ratio of 0.9:1 (zinc
nitrateþlanthanum nitrate:glycine) and stirred well for 1 h
in 100 ml double distilled water. The obtained solution is
heated ($185 1C) till combustion reaction occurs. Proce-
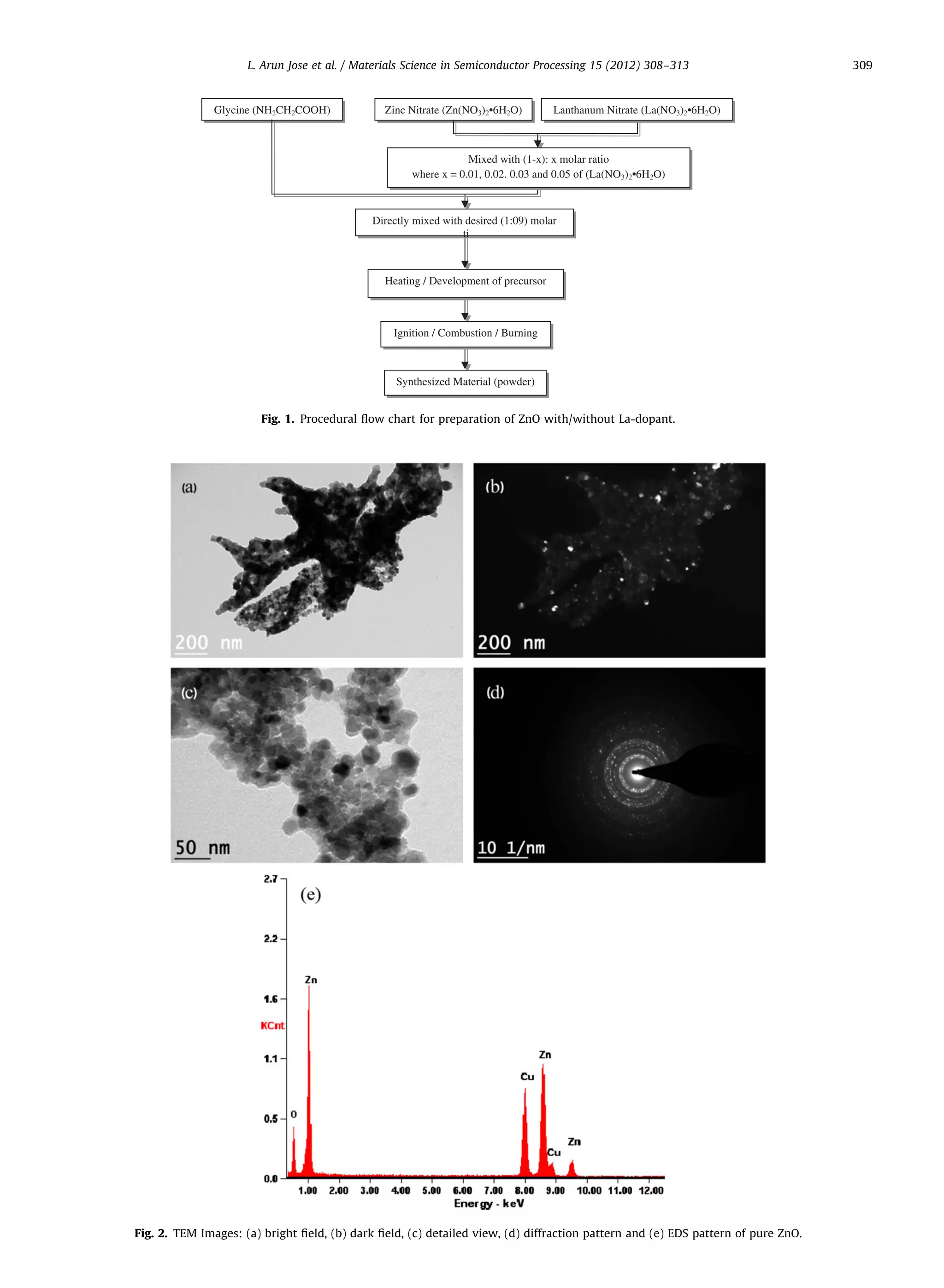
dural flow chart diagram for the preparation of precursors
and the formation of nano-structures is shown in Fig. 1.
Crystallinity of pure ZnO and La-doped ZnO catalysts were
analyzed by Philips CM 20 Transmission Electron Micro-
scope which was operated between 20 and 200 kV. Com-
position of the samples were analyzed by energy dispersive
X-ray spectroscopy (EDS) attached to the TEM instrument.
X-ray diffraction patterns of the synthesized samples were
Fig. 3. TEM images: (a) bright field, (b) dark field, (c) detailed view, (d) diffraction pattern and (e) EDS pattern of 5 mol % La-doped ZnO.
L. Arun Jose et al. / Materials Science in Semiconductor Processing 15 (2012) 308–313310](https://image.slidesharecdn.com/opticalstudiesofnano-structuredla-dopedznopreparedbycombustionmethod-160428034631/75/Optical-studies-of-nano-structured-la-doped-zn-o-prepared-by-combustion-method-3-2048.jpg)
![recorded using PAN analytical X-ray diffractometer with Cu
Ka (1.5405 ˚A) radiation in the scan range 2y between 301
and 701 with a scan speed of 21/min. UV–VIS spectra of pure
ZnO and La-doped ZnO catalysts were recorded using Varian
CARY 5E UV–VIS–NIR Spectrophotometer. The absorbance
spectra were then recorded in the range 200–700 nm.
Photoluminescence of pure ZnO and La-doped ZnO were
measured by Jobin Yvon Fluorolog spectrofluorometer and
the results are discussed in detail.
3. Results and discussion
TEM analysis shows that the nano-structures which
had been synthesized using combustion processing are
coral-shaped and porous as shown in Fig. 2. This shape
may be attributed to the thermal fluctuations while
synthesizing the samples. Grain size is found to be
$10–20 nm both in the case of pure and doped ZnO.
Porous nature of the nano-structures significantly increases
as the La-dopant concentration increases as shown in Fig. 3.
Each individual nano-structure is about 450–1000 nm
formed by tiny spherical ZnO nanoparticles. We can also
notice that the pores are $10–50 nm in diameter which
considerably increase the surface to volume ratio. Selected
area diffraction patterns match very well with wurtzite
ZnO in both pure and doped ZnO. EDS analysis shows that
some La3þ
ions have been incorporated into the ZnO lattice
by substituting zinc ions as shown in Fig. 3(e) and in
Table 1. When La is present the composition of oxygen
seems to be nearly constant. This may be due to the
addition of oxygen atoms in the La-doped ZnO which was
accommodated by the additional vacancy in the La3þ
ion.
Copper peak in the EDS measurement originates from the
TEM supporting carbon coated copper grid.
XRD profiles of synthesized pure and doped materials
in appropriate ratio are shown in Fig. 4. The diffraction
peaks and their relative intensities match with the JCPDS
card no. 36-1451. Hence the observed patterns can be
clearly endorsed to the presence of hexagonal wurzite
structure. XRD peak of lanthanum oxide was not observed
even for the La-doped sample with a high La concentra-
tion, suggesting that lanthanum oxide is uniformly dis-
persed in the ZnO and no second phase such as La2O3 and
La(OH)3 appears. It is evident that the introduction of La
ions does not alter the structure of ZnO and dopant
disperses homogeneously in the ZnO matrix as previously
reported [37]. Using the Scherrer equations the crystallite
sizes were estimated to be around 450 nm from the full-
width at half-maximum (FWHM) of diffraction peaks. The
diffraction pattern of ZnO is observed between the 2y
values of 301 and 701. The peak intensities of doped ZnO
increases with dopant concentration. Therefore, the crys-
talline nature of ZnO nanostructure increases with La-
dopant in the same manner as previously reported in the
case of Fe doped ZnO [38]. Doping of La ions restrains the
growth of ZnO grains and dopant with smaller ionic
radius has a constructive effect on diffusivity which
promotes orientation growth and good crystal [39]. The
lattice parameters and the unit cell volume were deter-
mined using software program UnitCell method of TJB
Holland & SAT Redfern [40]. The determined unit cell
parameters, volume and c/a were plotted as a function of
La concentrations and are shown in Figs. 5 and 6 respec-
tively. The lattice constant gradually increases with
increase in concentration of La3 þ
ions. Consequently, cell
volume and c/a ratio changed, agreeing with the fact that
ionic radii of La3 þ
is higher than the Zn2 þ
ion (0.106 nm
for La and 0.074 nm for Zn) [41,42] but there is a small
variation in c-axis compared with the results of Chen et al.
[37]. This distortion in the lattice parameters confirms the
incorporation of La3 þ
ions up to 5 mol% in ZnO wurzite
structure.
UV–VIS spectrum shows that the absorbance is high
below 380 nm for pure ZnO and as the La-dopant con-
centration increases the absorbance of ZnO decreases
considerably below this region as shown in Fig. 7. The
corresponding band gap values of pure and doped ZnO are
Table 1
Composition of elements in La-doped ZnO samples.
La concentration (mol%) Element weight (%) Atomic (%)
0 O 13.30 38.50
Zn 86.70 61.50
1 O 20.30 51.73
La 04.34 01.27
Zn 75.36 47.00
2 O 19.60 51.32
La 08.38 02.53
Zn 72.02 46.15
3 O 18.94 50.92
La 12.15 03.76
Zn 68.91 45.32
5 O 18.30 50.80
La 17.20 05.50
Zn 64.50 43.70
Fig. 4. Powder XRD spectra of samples pure–doped prepared at different
mol percent of La.
L. Arun Jose et al. / Materials Science in Semiconductor Processing 15 (2012) 308–313 311](https://image.slidesharecdn.com/opticalstudiesofnano-structuredla-dopedznopreparedbycombustionmethod-160428034631/75/Optical-studies-of-nano-structured-la-doped-zn-o-prepared-by-combustion-method-4-2048.jpg)
![presented in Fig. 8. It can be clearly seen that the band gap
of La-doped ZnO also increases gradually with increase in
La concentration. After 380 nm, absorbance of pure ZnO is
less compared with La-doped ZnO and absorbance
increases with increase in dopant concentration.
Photoluminescence (PL) spectra of La-doped ZnO
nano-structures were measured with an excitation wave-
length of 285 nm and is shown in Fig. 9. The intensity of
PL emission is found to increase with increase in La-
dopant, but the intensity of doped ZnO decreases in
comparison with pure ZnO between 3.2 and 3.3 eV. The
PL spectrum shows the La characteristic emission band at
$2.9 eV and near UV emission between 3.27 and 3.30 eV.
There is a shift in the emission spectra for pure and doped
ZnO. This may be attributed due to the strain created in
the crystal lattice to accommodate larger La atoms.
Spectra in the range of 340–460 nm (2.7–3.6 eV) shows
that a violet peak at about 420 nm (2.95 eV) and the
intensity of emission are found to be strongly reliant on
the La concentration. Traps on the grain surface per unit
volume increases with the increase of specific surface
area. Cordaro et al. [43] assumed that interface traps lie in
Fig. 7. UV–VIS spectra ZnO with/without dopant.
Fig. 8. Calculated band gap of pure and La-doped ZnO.
Fig. 5. Unit cell parameters a and c were plotted as a function of La
concentration.
Fig. 6. Unit cell volume and c/a were plotted as a function of La
concentration.
Fig. 9. Room temperature PL emission spectra of ZnO with/without
La-dopant.
L. Arun Jose et al. / Materials Science in Semiconductor Processing 15 (2012) 308–313312](https://image.slidesharecdn.com/opticalstudiesofnano-structuredla-dopedznopreparedbycombustionmethod-160428034631/75/Optical-studies-of-nano-structured-la-doped-zn-o-prepared-by-combustion-method-5-2048.jpg)
![the depletion regions and locate at the ZnO–ZnO grain
boundaries when a polycrystalline varistor forms, and the
level of interface trap was found to be about 0.33 eV
below the conduction band edge. So violet emission is
possibly attributed to the recombination centers linked
with interface traps existing at the grain boundaries, and
radiative transition occurs between the level of interface
traps and the valence band.
4. Conclusions
La-doped ZnO was prepared by combustion proces-
sing; doping levels included undoped, 1, 2, 3 and 5 molar
percentage. Significant transformation was observed upon
different doping concentrations. Transmission electron
micrograph shows an enhancement of pore density for
doped ZnO. Lattice parameters and unit cell volume were
determined from the XRD data and it confirms the entry
of La-dopant inside ZnO crystal lattice by the increase in
lattice constants. It is evident that the absorbance near UV
region decreases with increase in dopant concentration.
The bandgap is found to increase with addition of La. The
La-doped ZnO nano-structures prepared at low tempera-
tures are more suitable for applications such as chemical
and biological sensors, optoelectronic devices, and
solar cells.
Acknowledgments
The authors gratefully acknowledge BRNS (Board of
Research in Nuclear Sciences—Government of India, Pro-
ject no. 2008/37/12/BRNS/1513) for providing financial
assistance. They are also thankful to authorities of Indian
Institute of Technology, Chennai 36, for providing TEM,
UV–VIS, PL and powder XRD facility.
References
[1] J.B. Varley, A. Janotti, C. Franchini, C.G. Van de Walle, Physical
Review B 85 (2012) 081109. R.
[2] P.K. Sharma, R.K. Dutta, M. Kumar, P.K. Singh, A.C. Pandey, Journal
of Luminescence 129 (2009) 605–610.
[3] J. Kennedy, D.A. Carder, A. Markwitz, R.J. Reeves, Journal of Applied
Physics 107 (2010) 103518.
[4] H.-C. Shin, K.-R. Lee, S. Park, C.-H. Jung, S.-J. Kim, Japanese Journal of
Applied Physics 35 (1996) L996–L998.
[5] F. Li, K. Hu, J. Li, D. Zhang, G. Chen, Journal of Nuclear Materials 300
(2002) 82–88.
[6] L.E. Shea, J. McKittrick, O.A. Lopez, E. Sluzky, Journal of the
American Ceramic Society 79 (1996) 3257–3265.
[7] L. Chick, L. Pederson, G. Maupin, J. Bates, L. Thomas, G. Exarhos,
Materials Letters 10 (1990) 6–12.
[8] T. Mimani, K.C. Patil, Materials Physics and Mechanics 4 (2001)
134–137.
[9] R.D. Purohit, B.P. Sharma, K.T. Pillai, A.K. Tyagi, Materials Research
Bulletin 36 (2001) 2711–2721.
[10] B.D. Ahn, S.H. Oh, C.H. Lee, G.H. Kim, H.J. Kim, S.Y. Lee, Journal of
Crystal Growth 309 (2007) 128–133.
[11] H. Huang, Y. Ou, S. Xu, G. Fang, M. Li, X. Zhao, Applied Surface
Science 254 (2008) 2013–2016.
[12] V. Zhitomirsky, E. Cetinorgu, R. Boxman, S. Goldsmith, Thin Solid
Films 516 (2008) 5079–5086.
[13] T. Moriga, Y. Hayashi, K. Kondo, Y. Nishimura, K.-i. Murai,
I. Nakabayashi, H. Fukumoto, K. Tominaga, Journal of Vacuum
Science and Technology A 22 (2004) 1705.
[14] S. Saha, V. Gupta, AIP Advances 1 (2011) 042112.
[15] T.S. Herng, S.P. Lau, S.F. Yu, H.Y. Yang, K.S. Teng, J.S. Chen, Journal of
Physics: Condensed Matter 19 (2007) 356214.
[16] N.H. Hong, J. Sakai, V. Brize´, Journal of Physics: Condensed Matter
19 (2007) 036219.
[17] J. Zhang, X.Z. Li, J. Shi, Y.F. Lu, D.J. Sellmyer, Journal of Physics:
Condensed Matter 19 (2007) 036210.
[18] B. Li, X. Xiu, R. Zhang, Z. Tao, L. Chen, Z. Xie, Y. Zheng, Materials
Science in Semiconductor Processing 9 (2006) 141–145.
[19] T. Gao, T.H. Wang, Applied Physics A 80 (2004) 1451–1454.
[20] S. Anandan, A. Vinu, T. Mori, N. Gokulakrishnan, P. Srinivasu, V.
Murugesan, K. Ariga, Catalysis Communications 8 (2007)
1377–1382.
[21] Z.-X. Xu, V.A.L. Roy, P. Stallinga, M. Muccini, S. Toffanin, H.-F. Xiang,
C.-M. Che, Applied Physics Letters 90 (2007) 223509.
[22] C.-L. Hsu, T.-Y. Tsai, Journal of the Electrochemical Society 158
(2011) K20–K23.
[23] Y.R. Ryu, J.A. Lubguban, T.S. Lee, H.W. White, T.S. Jeong, C.J. Youn,
B.J. Kim, Applied Physics Letters 90 (2007) 131115.
[24] X.M. Teng, H.T. Fan, S.S. Pan, C. Ye, G.H. Li, Journal of Applied
Physics 100 (2006) 053507.
[25] S. Chirakkara, S.B. Krupanidhi, Physica Status Solidi RRL 6 (2012)
34–36.
[26] X. Chen, B. Xu, J. Xue, Y. Zhao, C. Wei, J. Sun, Y. Wang, X. Zhang,
X. Geng, Thin Solid Films 515 (2007) 3753–3759.
[27] P. Ruankham, T. Sagawa, H. Sakaguchi, S. Yoshikawa, Journal of
Materials Chemistry 21 (2011) 9710–9715.
[28] S. Mendoza-Galva´n, C. Trejo-Cruz, J. Lee, D. Bhattacharyya,
J. Metson, P.J. Evans, U. Pal, Journal of Applied Physics 99 (2006)
014306.
[29] I. Honma, S. Hirakawa, K. Yamada, J.M. Bae, Solid State Ionics 118
(1999) 29–36.
[30] M. Peres, A. Cruz, S. Pereira, M.R. Correia, M.J. Soares, A. Neves,
M.C. Carmo, T. Monteiro, A.S. Pereira, M.A. Martins, T. Trindade,
E. Alves, S.S. Nobre, R.A.Sa´ Ferreira, Applied Physics A 88 (2007)
129–133.
[31] S. Bachir, K. Azuma, J. Kossanyi, P. Valat, J.C. Ronfard-Haret, Journal
of Luminescence 75 (1997) 35–49.
[32] X.T. Zhang, Y.C. Liu, J.G. Ma, Y.M. Lu, D.Z. Shen, W. Xu, G.Z. Zhong,
X.W. Fan, Thin Solid Films 413 (2002) 257–261.
[33] G. Wu, Y. Zhuang, Z. Lin, X. Yuan, T. Xie, L. Zhang, Physica E
31 (2006) 5–8.
[34] S. Anandan, A. Vinu, K.L.P. Sheeja Lovely, N. Gokulakrishnan,
P. Srinivasu, T. Mori, V. Murugesan, V. Sivamurugan, K. Ariga,
Journal of Molecular Catalysis A: Chemical 266 (2007) 149–157.
[35] C. Ge, C. Xie, M. Hu, Y. Gui, Z. Bai, D. Zeng, Materials Science and
Engineering: B 141 (2007) 43–48.
[36] C.-C. Hwang, T.-Y. Wu, Journal of Materials Science 39 (2004)
6111–6115.
[37] J.T. Chen, J. Wang, F. Zhang, G.A. Zhang, Z.G. Wu, P.X. Yan, Journal of
Crystal Growth 310 (2008) 2627–2632.
[38] G.-Y. Ahn, S.-I. Park, S.-J. Kim, C.-S. Kim, Journal of Magnetism and
Magnetic Materials 304 (2006) e498–e500.
[39] S. Fujihara, C. Sasaki, T. Kimura, Journal of the European Ceramic
Society 21 (2001) 2109–2112.
[40] T.J.B. Holland, S.A.T. Redfern, Journal of Applied Crystallography 30
(1997) 84.
[41] S.H. Jeong, B.N. Park, S.B. Lee, J.H. Boo, Surface and Coatings
Technology 193 (2005) 340–344.
[42] Q. Yu, W. Fu, C. Yu, H. Yang, R. Wei, Y. Sui, S. Liu, Z. Liu, M. Li,
G. Wang, C. Shao, Y. Liu, G. Zou, Journal of Physics D: Applied
Physics 40 (2007) 5592.
[43] J.F. Cordaro, Y. Shim, J.E. May, Journal of Applied Physics 60 (1986)
4186.
L. Arun Jose et al. / Materials Science in Semiconductor Processing 15 (2012) 308–313 313](https://image.slidesharecdn.com/opticalstudiesofnano-structuredla-dopedznopreparedbycombustionmethod-160428034631/75/Optical-studies-of-nano-structured-la-doped-zn-o-prepared-by-combustion-method-6-2048.jpg)