1. A cylinder separated by an adiabatic piston initially contains nitrogen on one side and helium on the other at 20°C and 95 kPa.
2. Heat is added to the nitrogen side from a 500°C reservoir until the helium pressure reaches 120 kPa.
3. The final temperature of helium is calculated to be 321.7K, the final volume of nitrogen is 0.2838 m3, and the heat transferred to the nitrogen is 46.6287 kJ. The entropy generation is determined to be 0.057 kJ/K.






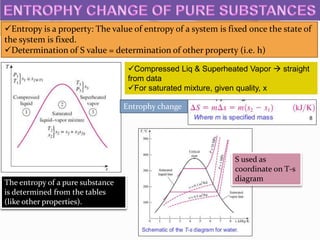






![Air is compressed from an initial state of 100kPa and 17°C to a final state of 600
kPa and 57°C. determine the entrophy change of air during this compression
process by using (a) property values from the air table (b) average specific heats
SOLUTION: Air is compressed between 2 specified states. The entrophy change is
to be determined by both method
ASSUMPTIONS: Air is an ideal gas
ANALYSIS: the initial & final states are fully specified
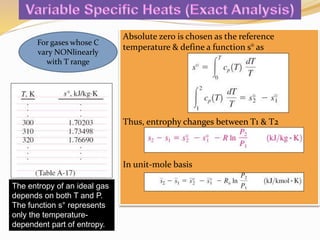
(a) Property table for s0 (Table A-17) & substituting into eq. for exact analysis [-
0.3844 kJ/kg.K]
(b) Find Cp values to determine the Cpave at Tave of 37°C (Table A-2b) and solve
using approximation analysis [-0.3842 kJ/kg.K]](https://image.slidesharecdn.com/week7entrophy-171207041313/85/Week-7-entrophy-15-320.jpg)












