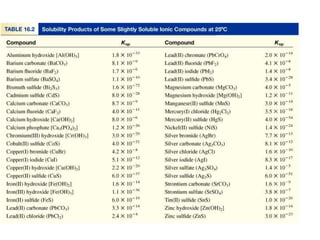
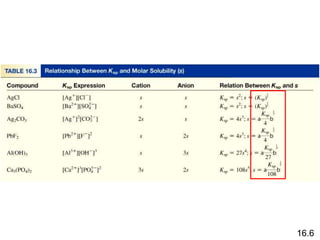
This document appears to contain information about solubility equilibria and the solubility product constant (Ksp). It provides examples of solubility equilibria expressions for binary and ternary salts dissolving in water. It also discusses how the value of Ksp relates to the solubility of salts, noting that Ksp values can only be used to directly compare the solubility of salts that contain the same number of ions. The document provides examples of calculating Ksp values from known solubility values and vice versa.




![Copyright © Cengage Learning. All rights reserved
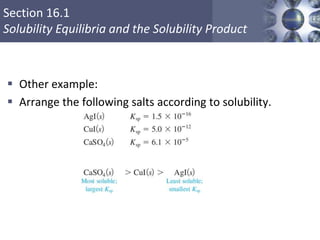
Some salts are less soluble in water. Much remains solid. The very
low water-soluble fraction of these salts, forms an equilibrium.
Solubility product (Ksp) – equilibrium constant; has only
one value for a given solid at a given temperature.
AgCl(s) Ag+(aq) + Cl–(aq)
Bi2S3(s) 2Bi3+(aq) + 3S2–(aq)
2 33+ 2
sp = Bi S
K
Ksp = [Ag+][Cl-]](https://image.slidesharecdn.com/chapter16-solubilityandcomplexionequilibria2-200610084629/85/Solubility-and-complex-ion-equilibria2-5-320.jpg)
![Section 16.1
Solubility Equilibria and the Solubility Product
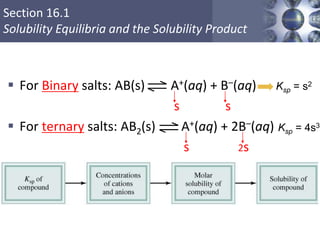
Examples:
MgF2 (s) Mg2+ (aq) + 2F- (aq) Ksp = [Mg2+][F-]2
Ag2CO3 (s) 2Ag+ (aq) + CO3
2- (aq) Ksp = [Ag+]2[CO3
2-]
Ca3(PO4)2 (s) 3Ca2+ (aq) + 2PO4
3- (aq) Ksp = [Ca2+]3[PO4
3-]2](https://image.slidesharecdn.com/chapter16-solubilityandcomplexionequilibria2-200610084629/85/Solubility-and-complex-ion-equilibria2-6-320.jpg)

![The solubility of calcium sulfate (CaSO4) is found to be
4.9 ×10-3 M. Calculate the value of Ksp for calcium sulfate.
CaSO4 (s) Ca2+ (aq) + SO4
2- (aq)
CaSO4 (s) Ca2+ (aq) + SO4
2- (aq) Ksp = [Ca2+][SO4
2-]
4.9 ×10-3 4.9 ×10-3
Ksp= (4.9 ×10-3)2= 2.4 ×10-5](https://image.slidesharecdn.com/chapter16-solubilityandcomplexionequilibria2-200610084629/85/Solubility-and-complex-ion-equilibria2-9-320.jpg)

![What is the solubility of silver chloride in g/L ?
AgCl (s) Ag+ (aq) + Cl- (aq)
Ksp = [Ag+][Cl-]Initial (M)
Change (M)
Equilibrium (M)
0.00
+s
0.00
+s
s s
Ksp = s2
s = Ksp
s = 1.3 x 10-5
[Ag+] = 1.3 x 10-5 M [Cl-] = 1.3 x 10-5 M
Solubility of AgCl =
1.3 x 10-5 mol AgCl
1 L soln
143.35 g AgCl
1 mol AgCl
x = 1.9 x 10-3 g/L
Ksp = 1.6 x 10-10
AgCl (s) Ag+ (aq) + Cl- (aq)](https://image.slidesharecdn.com/chapter16-solubilityandcomplexionequilibria2-200610084629/85/Solubility-and-complex-ion-equilibria2-13-320.jpg)

![Section 16.1
Solubility Equilibria and the Solubility Product
16
3s s
Ksp= [Ag+]3[PO4
3-]=27S4 S= 1.6×10-5 M
EXERCISE!
Ag3PO4 (s) 3Ag+ (aq) + PO4
3- (aq)
Calculate the solubility of silver phosphate in water.
Ksp = 1.8 × 10–18](https://image.slidesharecdn.com/chapter16-solubilityandcomplexionequilibria2-200610084629/85/Solubility-and-complex-ion-equilibria2-16-320.jpg)
