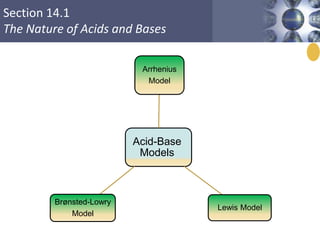
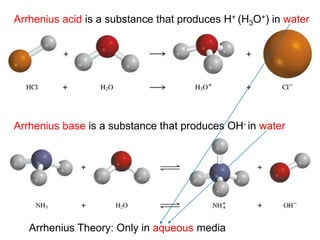
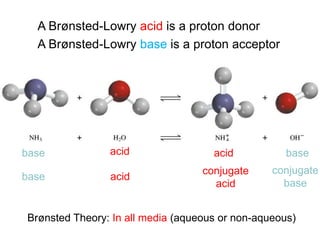
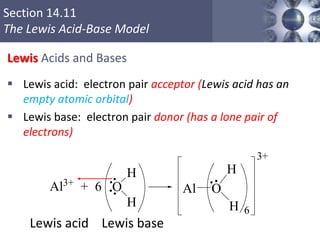
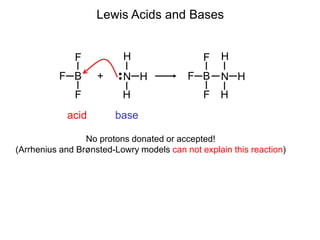
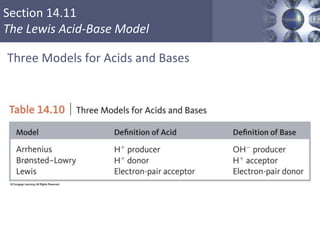
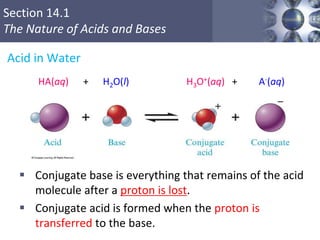
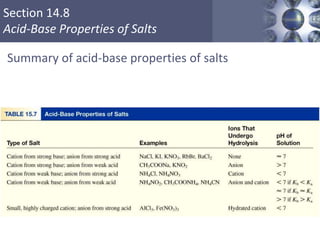
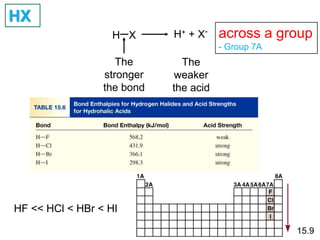
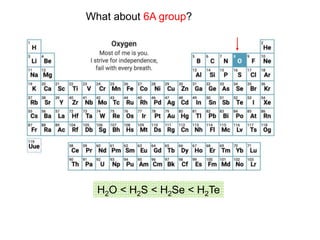
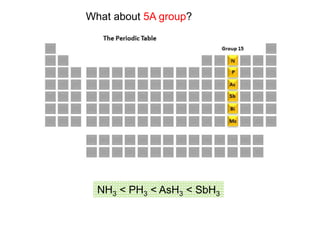
The document discusses acid-base models and the nature of acids and bases. It defines Arrhenius, Brønsted-Lowry, and Lewis acid-base theories. The Arrhenius model defines acids as hydrogen ion producers and bases as hydroxide ion producers in aqueous solutions. The Brønsted-Lowry model extends this to acid as a proton donor and base as a proton acceptor in any medium. The Lewis model defines acids as electron pair acceptors and bases as electron pair donors. The document also covers acid strength, pH and pOH scales, strong and weak acids/bases, and polyprotic acids.





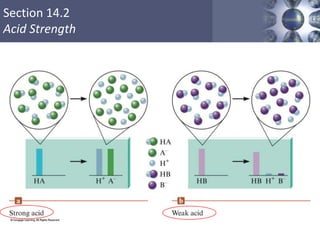



![Section 14.2
Acid Strength
Water is amphoteric:
Behaves either as an acid or as a base.
At 25°C:
H2O(l) + H2O(l) H3O+(aq) + OH-(aq)
Kw = [H+][OH–] = 1.0 × 10–14
Copyright © Cengage Learning. All rights reserved 19](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-19-320.jpg)

![Section 14.3
The pH Scale
pH = –log[H+]
Copyright © Cengage Learning. All rights reserved
21
pH is the measure of acidity.
[H+] = [OH-]
[H+] > [OH-]
[H+] < [OH-]
Solution Is
neutral
acidic
basic
[H+] = 1 x 10-7
[H+] > 1 x 10-7
[H+] < 1 x 10-7
pH = 7
pH < 7
pH > 7
At 250C
pH [H+]](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-21-320.jpg)

![Section 14.3
The pH Scale
Calculate the pH for each of the following solutions.
a) 1.0 × 10–4 M H+ b) 0.040 M OH–
Copyright © Cengage Learning. All rights reserved 23
EXERCISE!
a) pH = –log[H+] = –log(1.0 × 10–4 M) = 4.00
b) Kw = [H+][OH–] = 1.00 × 10–14 = [H+](0.040 M) = 2.5 × 10–13 M H+
pH = –log[H+] = –log(2.5 × 10–13 M) = 12.60](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-23-320.jpg)
![Section 14.3
The pH Scale
pH and pOH
Recall:
Kw = [H+][OH–]
–log Kw = –log[H+] – log[OH–]
pKw = pH + pOH
14.00 = pH + pOH
Copyright © Cengage Learning. All rights reserved 24](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-24-320.jpg)
![Section 14.3
The pH Scale
The pH of a solution is 5.85. What are the [H+] and [OH-]
for this solution?
Copyright © Cengage Learning. All rights reserved 25
EXERCISE!
[H+] = 10–5.85 = 1.4 × 10–6 M
pH + pOH=14
pOH=8.15
[OH-]= 10-8.15= 7.08×10-9](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-25-320.jpg)


![What is the pH of a 2 x 10-3 M HNO3 solution?
HNO3 is a strong acid – 100% dissociation.
HNO3 (aq) + H2O (l) H3O+ (aq) + NO3
- (aq)
pH = -log [H+] = -log [H3O+] = -log(0.002) = 2.7
Start
End
0.002 M
0.002 M 0.002 M0.0 M
0.0 M 0.0 M
What is the pH of a 1.8 x 10-2 M Ba(OH)2 solution?
Ba(OH)2 is a strong base – 100% dissociation.
Ba(OH)2 (s) Ba2+ (aq) + 2OH- (aq)
Start
End
0.018 M
0.018 M 0.036 M0.0 M
0.0 M 0.0 M
pH = 14.00 – pOH = 14.00 + log(0.036) = 12.6](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-32-320.jpg)

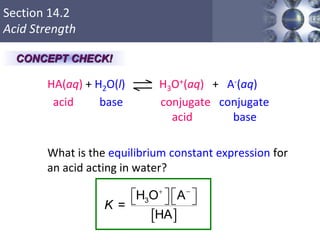
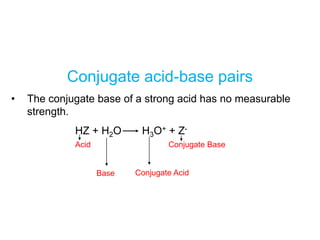
![HA (aq) + H2O (l) H3O+ (aq) + A- (aq)
HA (aq) H+ (aq) + A- (aq)
Ka =
[H+][A-]
[HA]
Ka is the acid ionization constant
Ka
weak acid
strength
15.5](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-34-320.jpg)
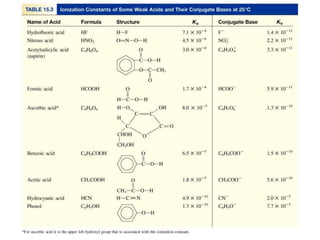
![What is the pH of a 0.5 M HF solution (at 250C)?
HF (aq) H+ (aq) + F- (aq) Ka =
[H+][F-]
[HF]
= 7.1 x 10-4
HF (aq) H+ (aq) + F- (aq)
Initial (M)
Change (M)
Equilibrium (M)
0.50 0.00
-x +x
0.50 - x
0.00
+x
x x
Ka =
x2
0.50 - x
= 7.1 x 10-4
Ka
x2
0.50
= 7.1 x 10-4
0.50 – x 0.50Ka << 1
x2 = 3.55 x 10-4 x = 0.019 M
[H+] = [F-] = 0.019 M pH = -log [H+] = 1.72
[HF] = 0.50 – x = 0.48 M](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-36-320.jpg)
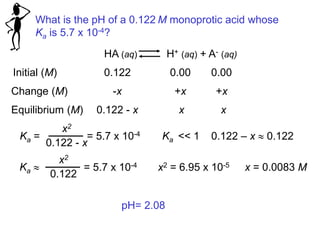

![Section 14.5
Calculating the pH of Weak Acid Solutions
For a given weak acid, the percent dissociation increases as the
acid becomes more dilute.
For example:
Percent ionization: HF(0.01 M)>HF(0.1 M)
39
amount dissociated (mol/L)
Percent dissociation = 100%
initial concentration (mol/L)
For a monoprotic acid HA
Percent ionization =
[H+]
[HA]0
x 100% [HA]0 = initial concentration
Weak Acids (HA) not completely dissociate](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-39-320.jpg)
![Section 14.5
Calculating the pH of Weak Acid Solutions
A solution of 8.00 M formic acid (HCHO2) is 0.47%
ionized in water. Calculate the Ka value for formic acid.
40
EXERCISE!
Ka = = 1.8 × 10–4
[H+][HCO2-]
[HCHO2]
If 8.00 M of the acid is 0.47% ionized, then 0.038 M dissociates.
Ionized concentration: [HCHO2] = 0.47×0.08= 0.038
HCHO2(aq) + H2O H3O+(aq) + CHO2
-(aq)
I 8.00 0 0
C -0.038 +0.038 +0.038
E 7.96 0.038 0.038](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-40-320.jpg)
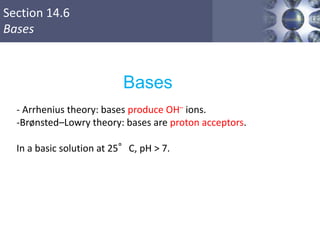
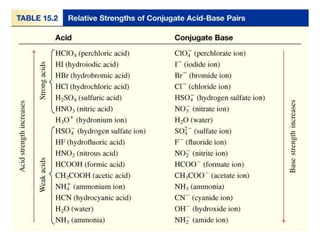
![Section 14.6
Bases
Ionic compounds containing OH- are generally
considered strong bases.
LiOH, NaOH, KOH, Ca(OH)2, Sr(OH)2, Ba(OH)2
pOH = –log[OH–]
pH = 14.00 – pOH
Copyright © Cengage Learning. All rights reserved 42
Strong bases-100% dissociation](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-42-320.jpg)

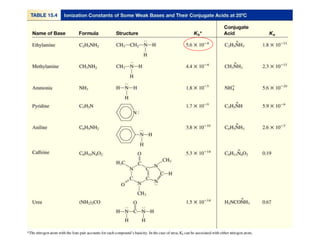
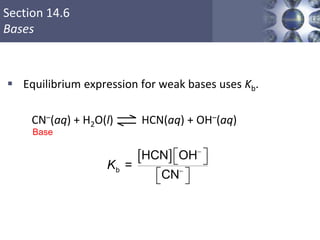
![NH3 (aq) + H2O (l) NH4
+ (aq) + OH- (aq)
Kb =
[NH4
+][OH-]
[NH3]
Kb is the base ionization constant
Kb
weak base
strength
Solve weak base problems like weak acids
except solve for [OH-] instead of [H+].](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-46-320.jpg)
![Section 14.6
Bases
pH calculations for solutions of weak bases are very
similar to those for weak acids.
Kw = [H+][OH–] = 1.0 × 10–14
pOH = –log[OH–]
pH = 14.00 – pOH
Copyright © Cengage Learning. All rights reserved 47](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-47-320.jpg)


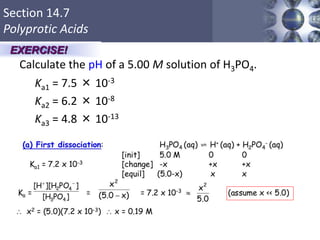



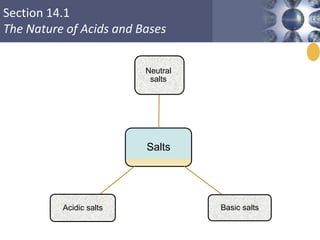

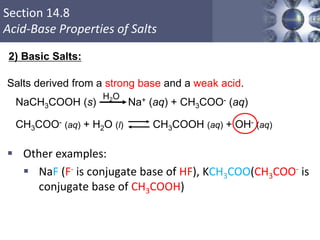
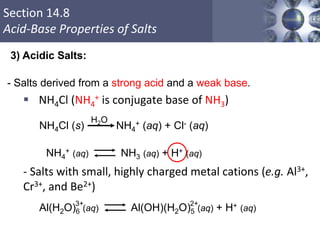


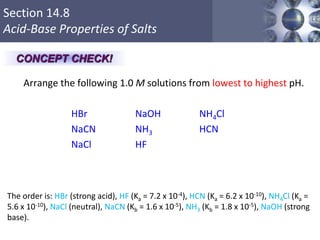

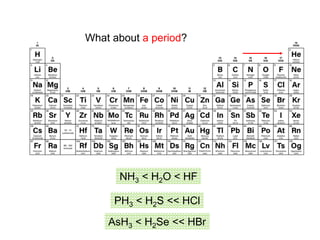

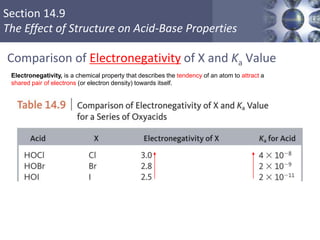


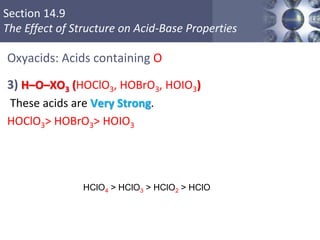

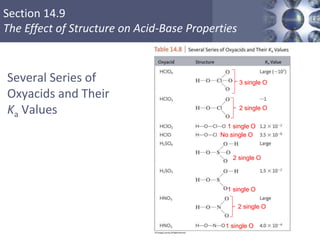



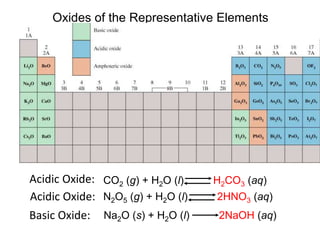
![What is amphoteric oxides
an amphoteric compound is a molecule or ion that can react
both as an acid and as a base. Al2O3 is an example of an
amphoteric oxide.
•In acid: Al2O3 + 6 HCl→ 2 AlCl3 + 3 H2O
•In base: Al2O3 + 2 NaOH + 3 H2O → 2 Na[Al(OH)4]](https://image.slidesharecdn.com/chapter14-acidsandbases-200610084433/85/Acids-and-bases-78-320.jpg)
