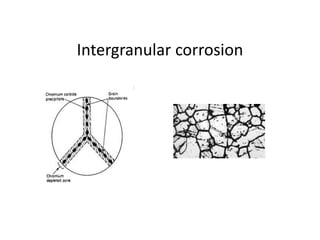
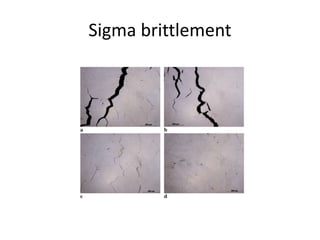
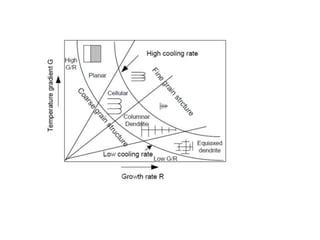
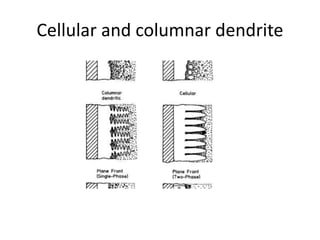
The document discusses the weldability of various stainless steel types, including austenitic, ferritic, and martensitic stainless steels. It provides information on their typical compositions and applications. It also describes various welding techniques that can be used and issues that may occur during welding like sensitization, sigma phase formation, and hydrogen cracking. Prevention methods are outlined like using stabilizers, annealing treatments, and controlling cooling rates and heat inputs during welding.