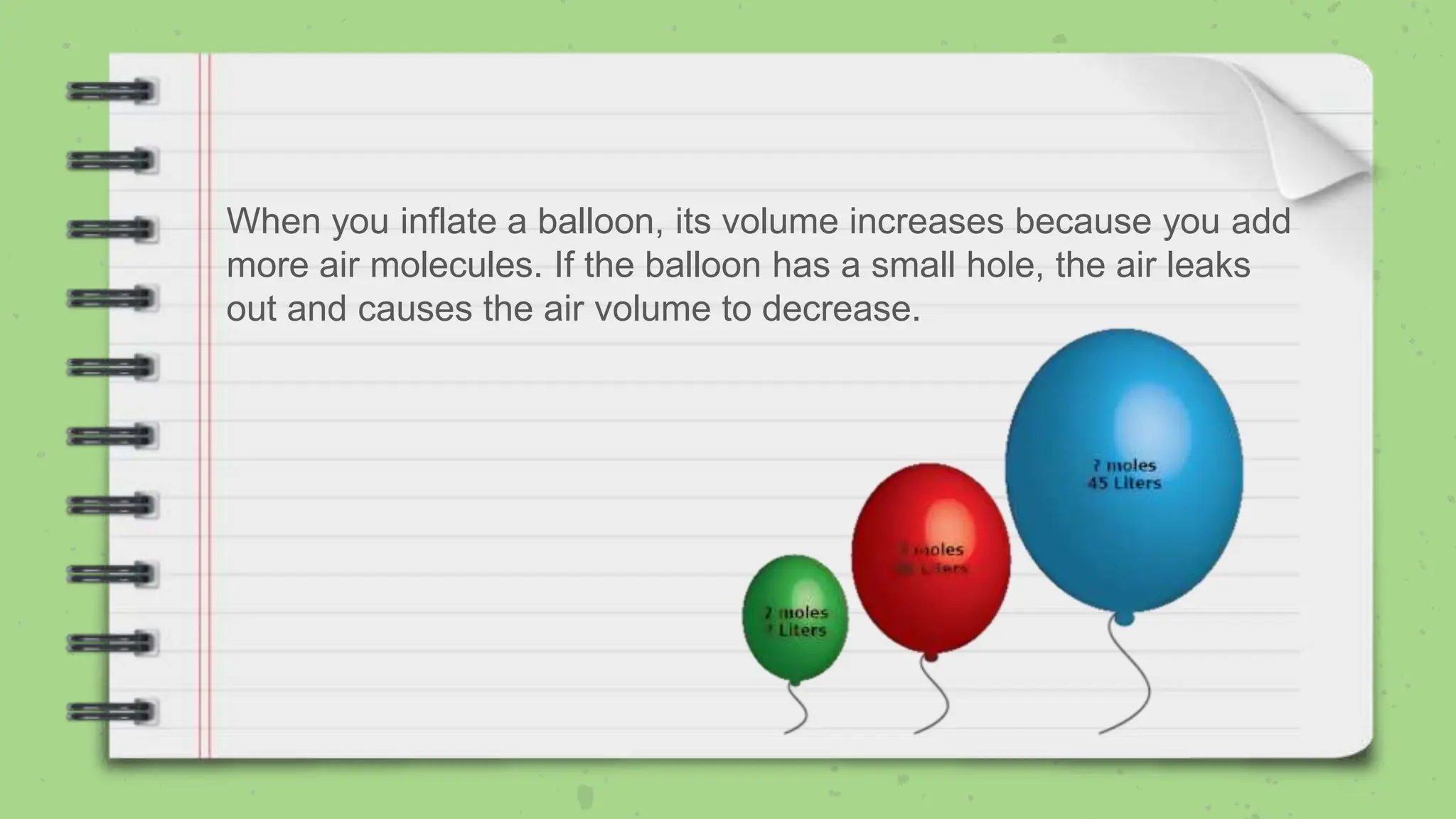
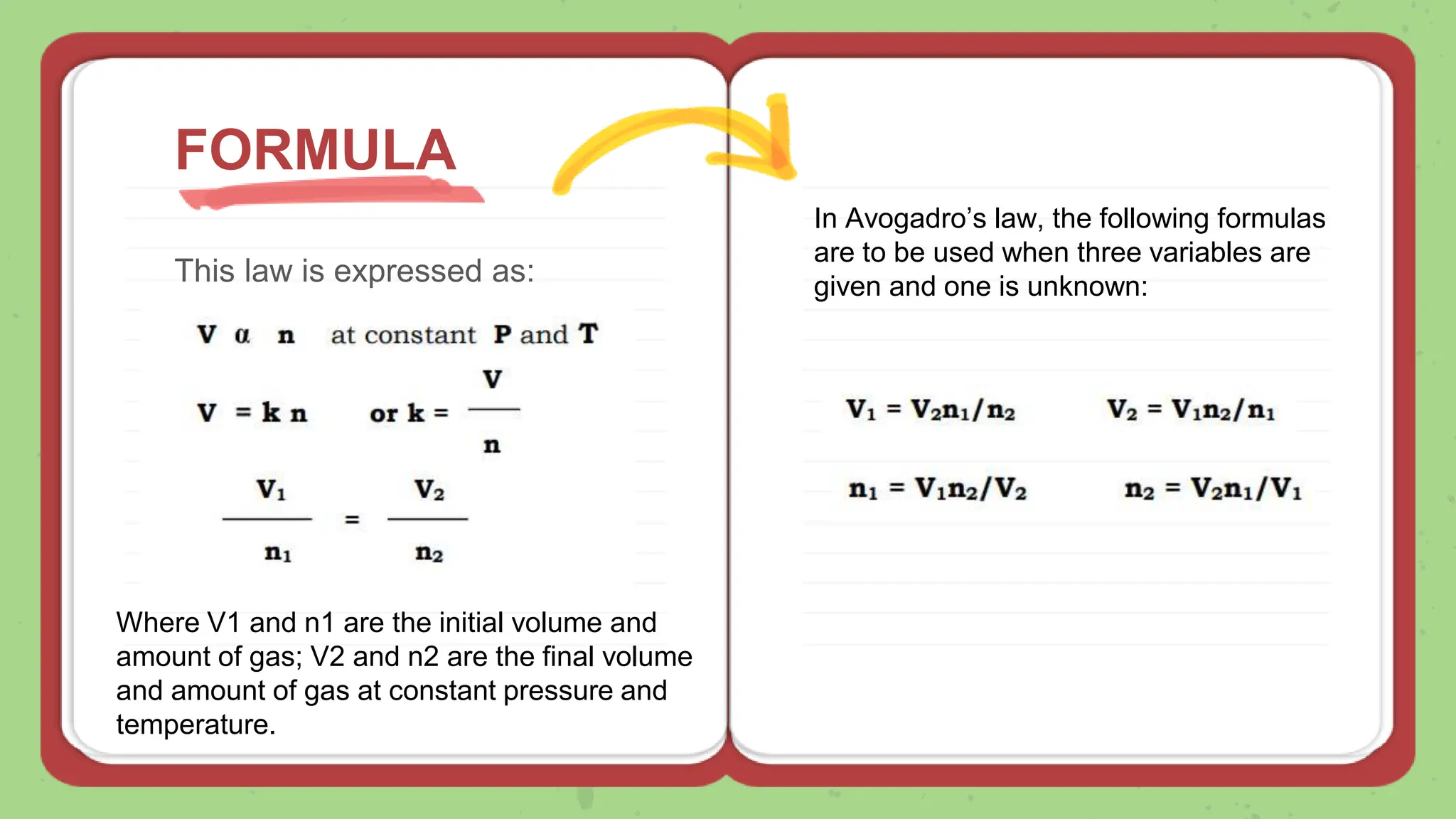
Avogadro's law states that the volume of a gas at constant temperature and pressure is directly proportional to the number of moles of gas present. The document explains the law using examples and formulas to calculate unknown variables with given values. It also includes problems for practice related to the application of the law.