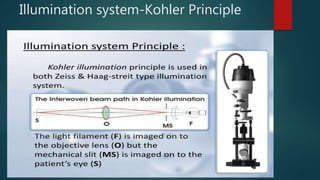
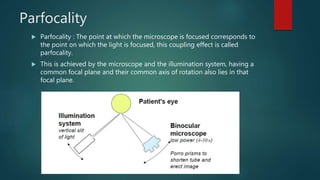
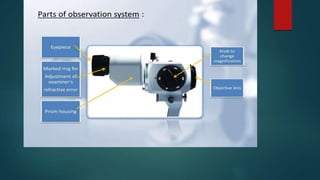
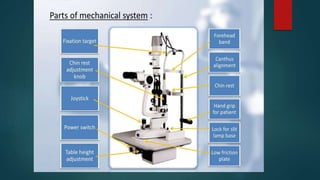
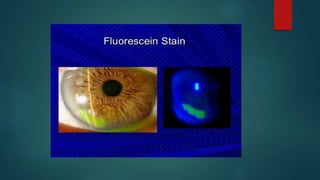
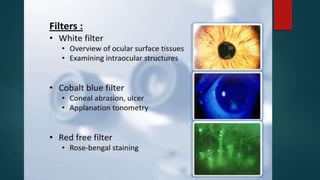
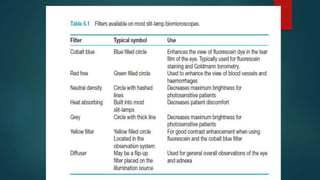
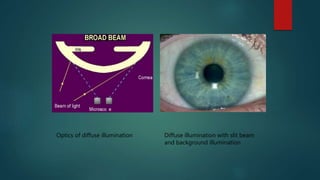
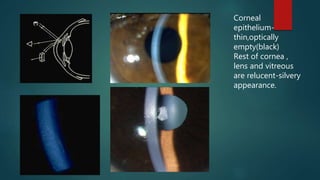
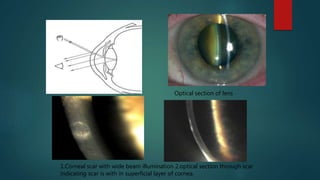
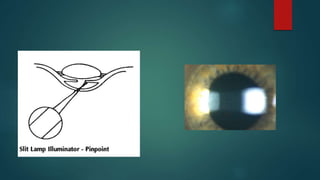
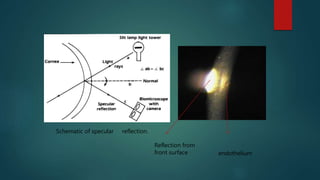
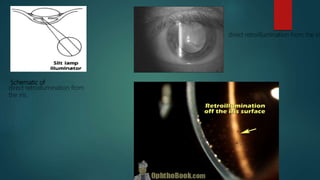
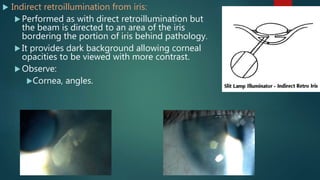
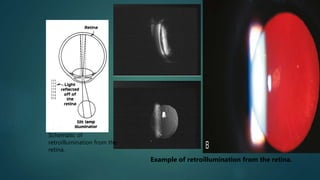
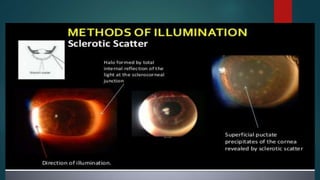
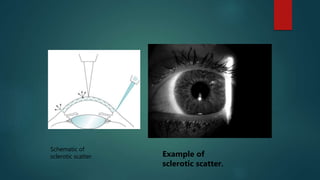
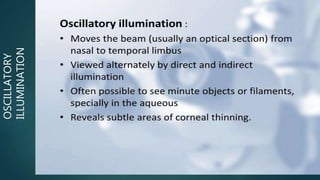
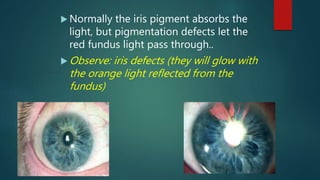
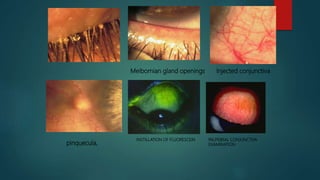
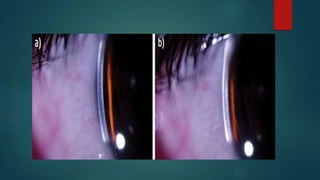
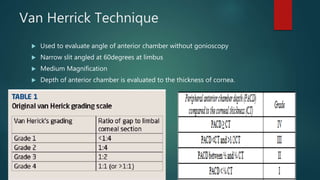
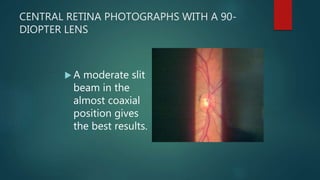
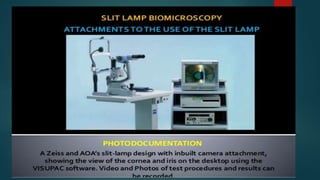
The slit lamp biomicroscope allows high-powered, stereoscopic examination of the eye. It has two main components: an illumination system that produces a thin slit of light using the Kohler principle, and an observation system consisting of binocular microscopes. There are two common types - Zeiss and Haag Streit - which differ in the position of the light source. Various illumination techniques like diffuse, direct, retro-illumination and specular reflection are used to visualize different ocular structures. The slit lamp enables in-vivo examination of the anterior segment in 3D and is invaluable for diagnostic and surgical procedures.