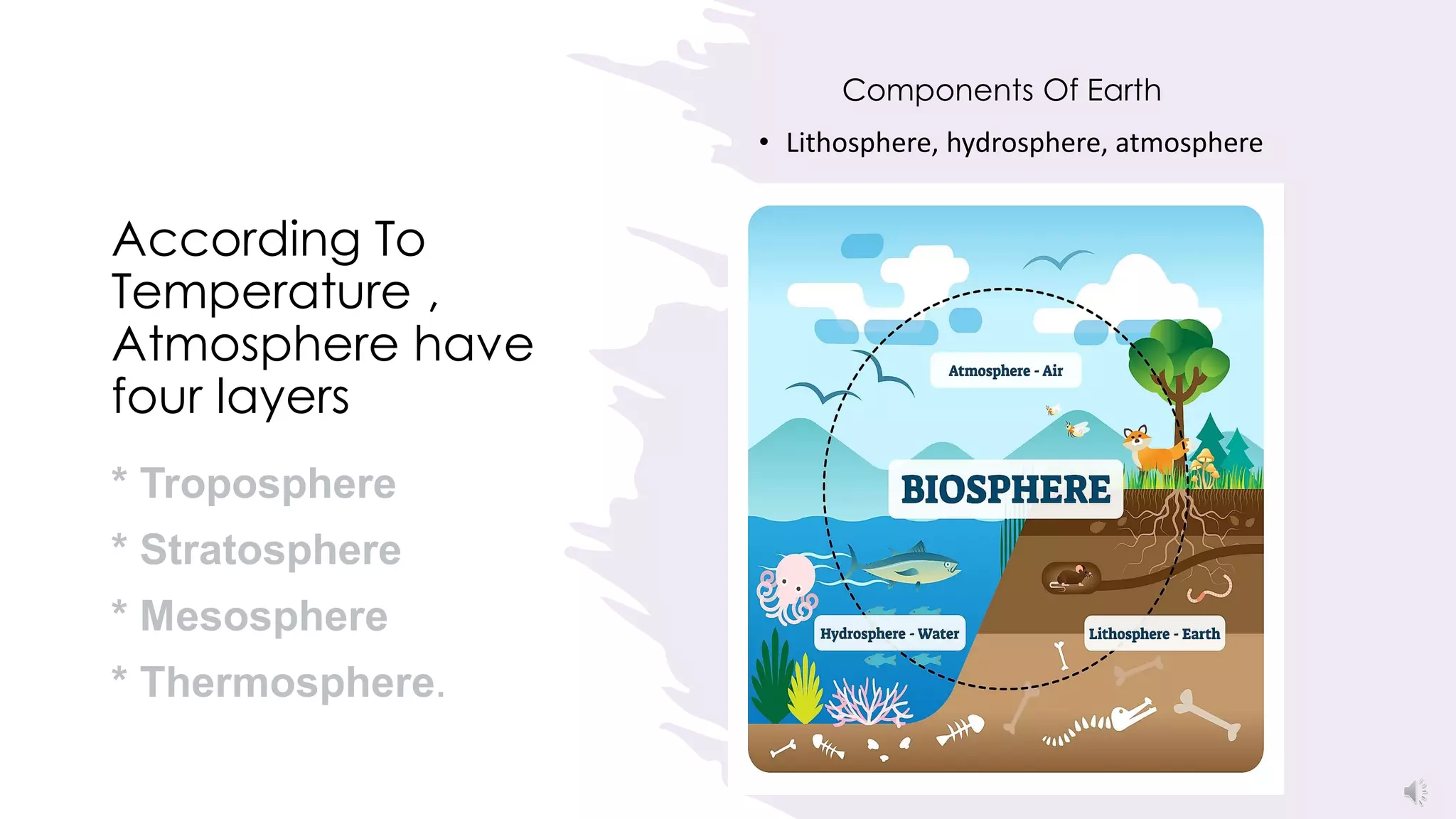
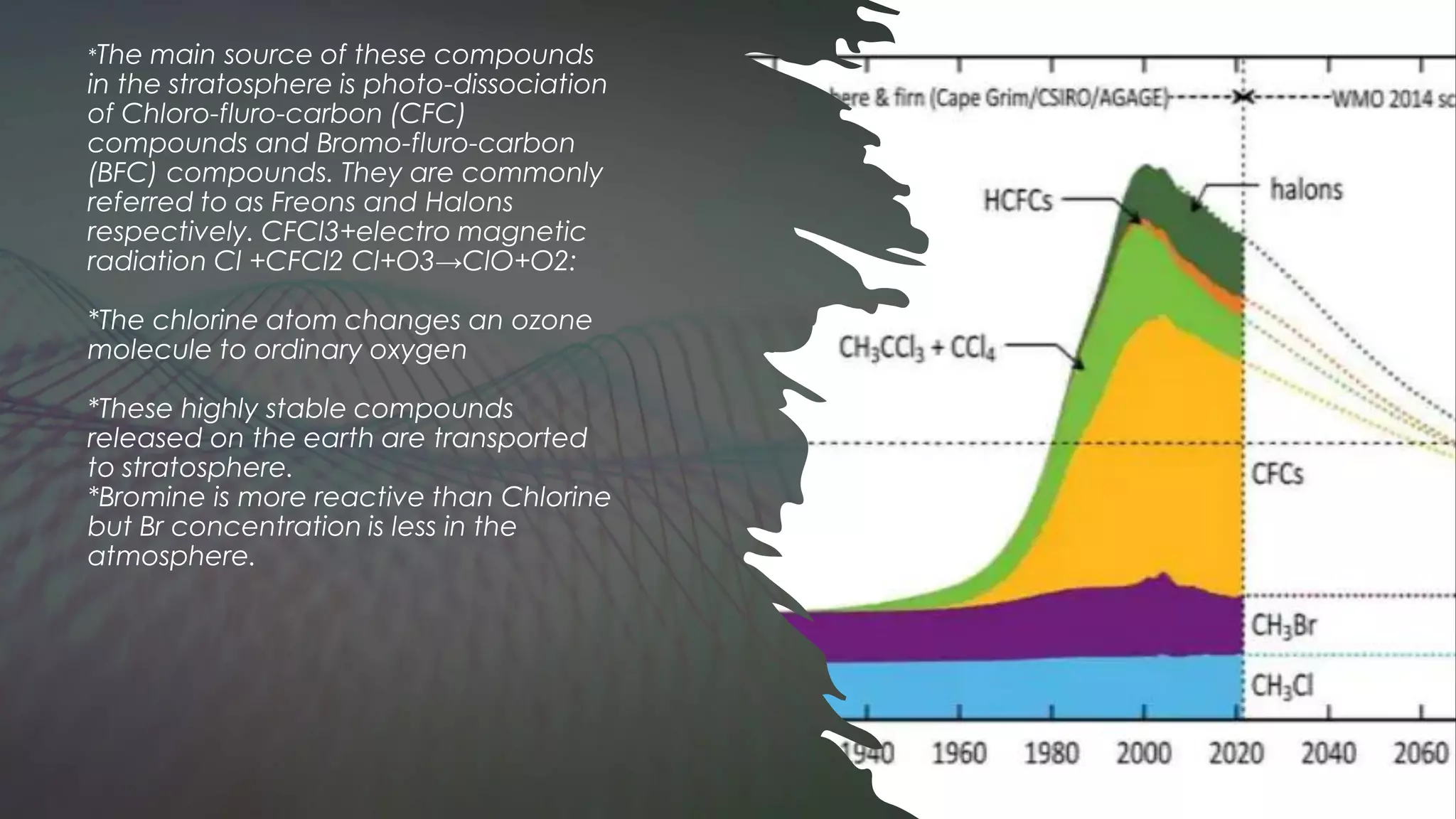
The Montreal Protocol has successfully protected the ozone layer by phasing out ozone-depleting substances, allowing the ozone hole to heal. World Ozone Day, celebrated each year on September 16, highlights the continued importance of the Montreal Protocol in protecting human health, economies, ecosystems and the climate from ultraviolet radiation. This year's theme emphasizes how the Montreal Protocol has kept vaccines cool and effective during distribution by curbing gases harmful to the ozone layer and climate. The ozone layer is vital as it filters out much of the sun's harmful ultraviolet rays, and the Montreal Protocol's phase-out of chlorofluorocarbons and other ozone-depleting substances has allowed the ozone layer to begin recovering