Embed presentation
Downloaded 129 times











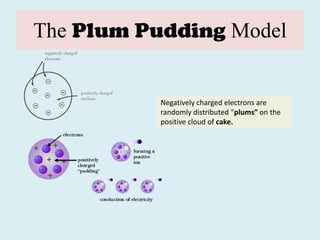


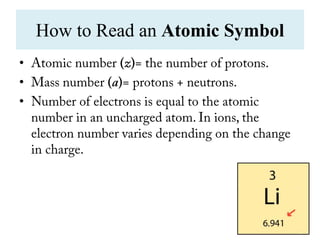


The document traces the evolution of the atomic theory from ancient Greek observations to the modern quantum mechanical model. It describes key discoveries and concepts such as Dalton's atomic theory, Avogadro's hypothesis, the discovery of the electron, Thomson's plum pudding model of the atom, Rutherford's nuclear atom model, and the development of quantum mechanics in the early 20th century which established that atoms and electrons behave as both particles and waves.























