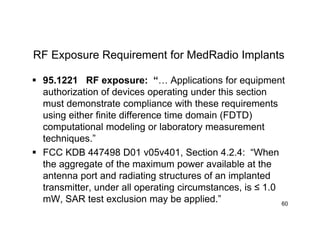
The document discusses wireless trends in medical devices and technologies. It covers increasing wireless approvals, regulatory changes, new standards, and emerging wireless medical technologies including wearables, inductive devices, medical body area networks (MBAN), and wireless medical telemetry service (WMTS). Risks of wireless medical devices are also mentioned.













![FCC Report and Order (FCC 14-208)
FCC will no longer accept applications for certification. All grants will be
issued by TCBs.
The Permit But Ask procedure will be codified.
Clarification of TCB responsibilities for performing market surveillance.
Specify steps for addressing instances of deficient TCB performance,
including sanctions and rescinding TCB authority.
Modify the rules to reference new standards (ISO/IEC 17065) used to accredit
TCBs
Require labs that test for certification or DoC to be accredited. [“Testing
laboratories located in non MRA countries are not FCC recognized as
accredited under the current procedures.“]
Codify a procedure through which the Commission currently recognizes new
laboratory accreditation bodies;
Update references to industry measurement procedures in the Commission’s
rules (e.g. ANSI C63.4-2014 and ANSI C63.10-2013); and
Provide greater flexibility to enable the FCC’s OET to address minor technical
issues that may be raised when updating to the latest versions of industry
standards that are referenced in Parts 2, 5, 15, and 18 of the Commission’s
rules.
16](https://image.slidesharecdn.com/testingwirelessmedicaldevices-gregkiemel-5-8-2015-151229194509/85/Testing-wireless-medical-devices-16-320.jpg)