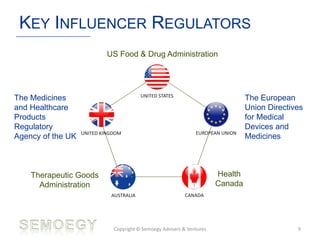
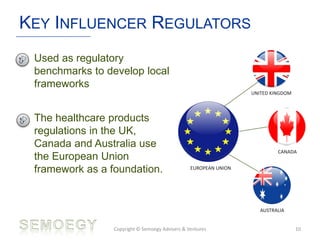
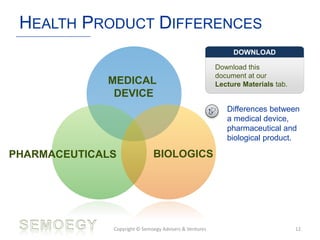
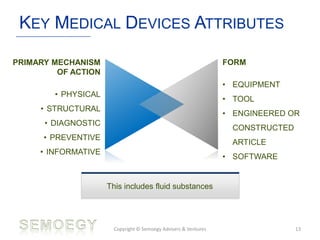
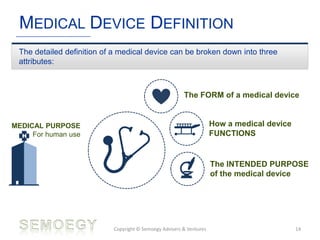
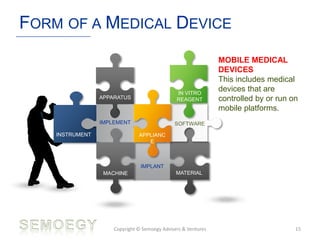
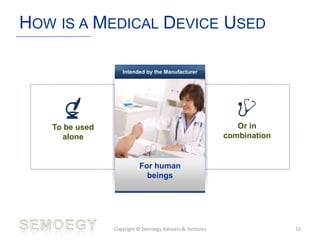
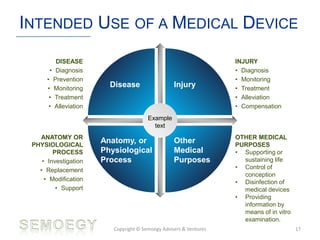
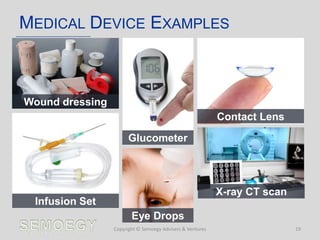
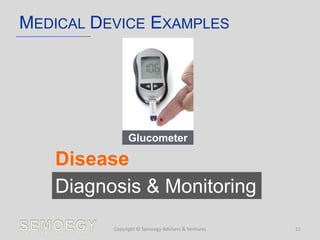
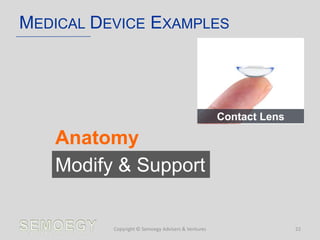
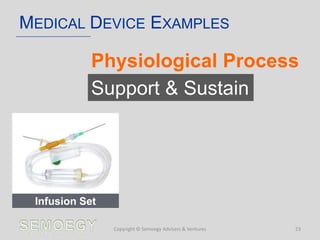
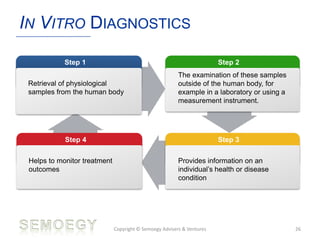
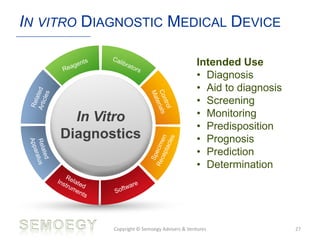
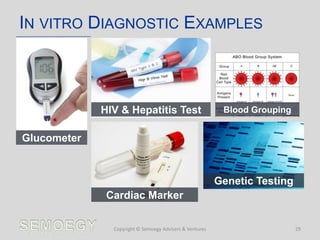
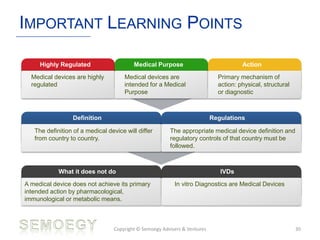
The document provides an overview of medical technology education, focusing on the definition and regulation of medical devices. It details the attributes and intended purposes of medical devices, as well as the regulatory frameworks across various countries. Additionally, it highlights the distinction between medical devices, pharmaceuticals, and biologics, and includes examples of medical devices and their functions.