This document discusses the evolution and functions of the temporal lobe. It notes that the temporal lobe is larger in humans compared to apes, with increased gyral white matter and interconnectivity. This supports the development of social cognition abilities in humans. The temporal lobe, and specifically the amygdala, plays a key role in social processing, interpreting facial expressions and eye gaze. Damage to the temporal lobe can cause a variety of cognitive, behavioral and neurological symptoms, depending on whether the left or right lobe is affected. Syndromes like Geschwind-Gastaut syndrome and Kluver-Bucy syndrome may result from right temporal lobe lesions.
![83
© Springer International Publishing Switzerland 2016
M. Hoffmann, Cognitive, Conative and Behavioral Neurology,
DOI 10.1007/978-3-319-33181-2_5
Temporal Lobe Syndromes
5
Evolution of the Temporal: Some
Pertinent Details
This is one of the few cortical regions that are larger
in size in humans in comparison studies with apes.
Overall there is a relative white matter increase but
with a specific gyral white matter increase as
opposed to core white matter which is not rela-
tively increased. Gyral white matter increase as
opposed to core white matter is thought to mediate
much greater interconnectivity, enabled by the
short association fibers [1]. In comparative analy-
ses, amongst modern humans, relatively wider
orbitofontal cortices, enlarged olfactory bulbs, and
larger and more forwardly placed temporal lobe
poles are evident, consistent with social brain
development [2]. The amygdaloid complex is par-
ticularly concerned with the social brain develop-
ment such as social cognition, coalitions, and
emotional regulation. Another unique human
development is the relative enlargement of the
basolateral nucleus of the amygdaloid group of
nuclei (lateral, basal, accessory nuclei). This is also
consistent with the general surge in interconnectiv-
ity of the temporal lobe association cortices [3, 4].
The Evolutionary Importance
of the Social Circuitry
and the Social Brain
Hypothesis
The importance of social cohesiveness and the
challenge of the polyadic relationships are con-
sidered to be a major if not key drivers of
increasing human brain size (Fig. 5.1) [5]. With
the temporal lobe a central component of social
processing with the amygdala in particular it is
not surprising that some significant human
enlargements have been reported in this part of
the brain. Human gaze and eye contact are
important initial contact modes and ascertaining
eye gaze direction of a conspecific or other
human and its monitoring are functions of supe-
rior temporal lobe [6]. The amygdala also has a
key role in interpreting social facial signals
from the face. A functional MRI study revealed
that during eye contact (and also without eye
contact) the direction of gaze activated the left
amygdala indicating a general role in monitor-
ing eye gaze. This differed from the right side
where only during eye contact was the right
amygdala activated [7].](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-1-320.jpg)
![84
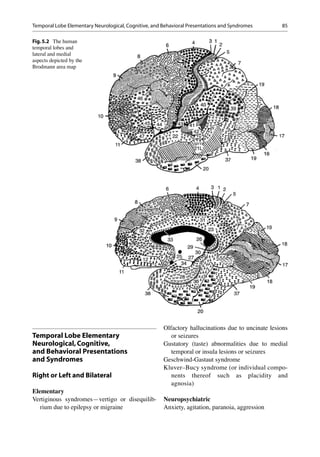
Neuroanatomy and Neurophysiology
The anatomical confines of the temporal lobes
include (Fig. 5.2):
Lateral
Heschl’s gyrus, planum polare, planum tempo-
rale (BA 41, 42, 22)
Superior temporal middle and inferior temporal
gyrus (BA 22, 21, 20)
Medial
Inferior temporal gyrus (BA 20)
Parahippocampal gyrus (BA 27, 28, 34, 35)
Fusiform gyrus (BA 36)
The posterior temporal lobes are delimited by
an arbitrary line drawn from the parietooccipital
sulcus to the preoccipital notch (indentation in
the inferior temporal gyrus). A horizontal line,
drawn from the midpoint of this particular line to
the lateral sulcus, demarcates the temporal and
parietal lobes [8].
The temporal lobes are intimately tied to all
the other lobes through the association tracts.
Amongst the largest long-range association tracts
include the occipitotemporal and the uncinate
fasciculus. Anterior temporal lobe, inferior fron-
tal lobe lesions, and those of the uncinate fascicu-
lus may be affected together by lesions or disease
states with difficulty in parsing out which is the
most responsible. Accordingly some authors
regard syndromes of the uncinate fasciculus as an
appropriate approach.
Sensory visual and auditory (much less olfac-
tory) inputs mediate evaluation of a conspecific’s
or other human’s eyes, faces, and body move-
ment. Specialized and separate temporal cortical
areas have been identified for these. Supportive
evidence comes from lesion studies as well as
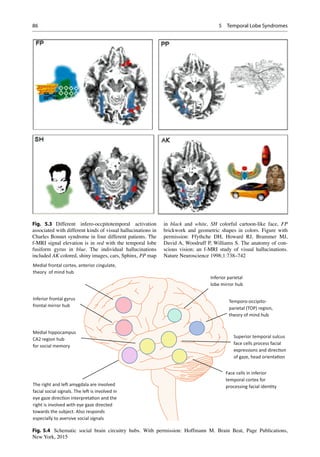
functional imaging studies with fMRI. The study
by ffytche et al. demonstrated the parts activated
during visual hallucinations for faces, places, and
objects (Fig. 5.3) [9]. These are then subsequently
relayed to superior temporal gyrus and amygdala
for salience evaluation. The mirror neuron cir-
cuitry is concerned with theory of mind detection.
In addition the social semantic memory of the
anterior temporal lobe for faces for example forms
part of the social circuitry (Fig. 5.4).
Fig 5.1 The social
brain hypothesis: group
size for primates and
humans and neocortex
ratio. Index of relative
cortex size (neocortex
ratio) is neocortex
volume divided by the
volume of the rest of the
brain. Figure with
permission: Gamble C,
Gowlett J, Dunbar
R. Thinking Big. How
the evolution of social
life shaped the human
mind. Thames and
Hudson, London 2014
5 Temporal Lobe Syndromes](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-2-320.jpg)
![87
Cognitive
Memory: Korsakoff amnestic state
Cortical deafness
Auditory agnosia (inability to identify sounds
despite normal peripheral hearing status)
Auditory paracusias
Auditory hallucinations (simple and complex),
illusions (differentiate from peduncular
hallucinosis)
Disorders of time perception (time may pass with
excessive speed or not at all)
Left
Elementary
Right upper quadrantanopia
Cognitive
Aphasias: Wernicke’s, transcortical sensory, and
anomic
Memory: Verbal amnesia
Visual agnosia
Amusia: Lexical amusia (impairment in reading
music)
Synesthesia
Right
Elementary
Left upper quadrantanopia
Cognitive
Memory: Visuospatial amnesia
Prosopagnosia (occipital–temporal region)
Auditory agnosia—verbal (pure word deafness)
and nonverbal (environmental sounds)
Amusias—receptive and expressive
Delusional misidentification syndromes
Theory of mind impairment (semantic dementia)
[10–17]
Neuropathological Processes
The more commonly encountered pathologies
that involve the temporal lobe in relative isolation
include inferior division middle cerebral artery
territory bland infarction, intracerebral hemor-
rhage (Fig. 5.5), epilepsy, encephalitis, tumors,
and traumatic brain injury. Aside from the apha-
sic presentations that are typical of left temporal
lobe involvement, right temporal lobe syndromes
may be more enigmatic or covert. These include
Kluver–Bucy syndrome (KBS) and Geschwind-
Gastaut syndrome (GGS) presentations or frag-
ments thereof or forme fruste varieties. The KBS,
originally described in monkeys, is rare and gen-
erally ascribed to bilateral lesions although cases
have been reported with unilateral lesions [18].
The presentation includes some or all of the
following:
• Visual agnosia
• Hyperorality
• Placidity
• Altered sexual activity both hypersexuality or
hyposexuality
• Hypermetamorphosis
Human forms of the KBS are being increas-
ingly described with manifestations such as com-
pulsive social kissing reported in a person with
Fig. 5.5 Isolated, discrete, right temporal lobe intracere-
bral hemorrhage (arrow)
Temporal Lobe Elementary Neurological, Cognitive, and Behavioral Presentations and Syndromes](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-5-320.jpg)
![88
frontotemporal lobe dementia [19], hypersexual-
ity, and hyperphagia post-right temporal lobec-
tomy for seizure management [20]. KBS may be
permanent or transient and has been reported in
TBI, ICH, FTD, and infectious such as herpes
simplex encephalitis TBI and KBS [21, 22].
Geschwind-Gastaut Syndrome
Although the GGS had for many years been
described in the context of temporal lobe epilepsy,
specifically the interictal phase [23, 24], both iso-
lated bland infarcts and intracerebral hemorrhage
and so the right temporal lobe in particular have
been correlated with this syndrome. The right
temporal lobe had been regarded as one of the so-
called silent areas of the brain but in addition to
the GGS, delusional misidentification syndromes
and a variety of accompanying frontal network
syndromes are frequently encountered with
lesions of this area, if tested for. Importantly these
complex syndromes generally occur without her-
alding sensorimotor deficits (Fig. 5.6) [25].
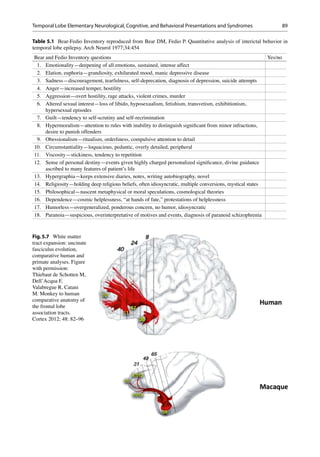
This syndrome is comprised of three core fea-
tures; the diagnostic process is facilitated by the
Bear-Fedio Inventory (Table 5.1):
1. Viscous personality
2. Metaphysical preoccupation
3. Altered physiological drives.
The viscous personality may be regarded as the
key component of the GG syndrome and may
incorporate one or more of the following features:
• Circumstantiality in discourse
• Overinclusive or excessively detailed narrative
information
• Excessive detail may present with hypergraphia,
painting, drawing
• Undue prolongation of the interpersonal
exchange [26]
Metaphysical Preoccupation
1. Incipient and intense intellectual pursuits per-
taining to morality, religion, and philosophy.
Other behavioral and physiological deviations
such as hyposexuality or hypersexuality, undue
fear, or aggression [23, 24, 27].
Uncinate Fasciculus
The UF is a late-maturing (third–fourth decades),
major brain long-range association fiber tract
connecting the OFC and anterior temporal lobes
(Fig. 5.7). Of note is that it is particularly vulnerable
to traumatic brain shearing-type injury. Its late
maturation makes it a site for neuropsychiatric
Fig.5.6 Isolated right
frontal temporal
encephalomalacia
(arrows) post-
hemorrhage and
craniotomy in person
with classic Geschwind-
Gastaut syndrome
5 Temporal Lobe Syndromes](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-6-320.jpg)
![90
syndromes affecting young adults [28]. Three
principal neurophysiological functions of the UF
include episodic memory, social emotional, and
linguistic processing. Pathological states linked
to UF injury include the following:
Social-Emotional Processing
Impairment
The anterior temporal pole is the site for storage
as well as retrieval of socially related memories
and person-related memories and a hub for the
theory of mind circuitry.
Uncinate Fits
These are dreamy states, involving olfactory
and/or gustatory hallucinations, sexual and other
emotional arousals, and involuntary facial or oral
activities [29].
Delusional Misidentification
Syndromes
The UF is the site of injury at times for the delu-
sional misidentification syndromes such as those
with Capgras delusions indicate an impairment in
the face-processing cortical regions to limbic
areas that convey emotional salience or valence
to the faces [30–32].
Cortical Deafness
Due to bilateral lateral temporal lobe lesions
involving Heschl’s gyrus very similar hearing
abnormalities may occur with brainstem stroke due
to anterior inferior cerebellar artery occlusion [33].
Auditory Agnosia
An inability to identify sounds that may be either
verbal (pure word deafness) or nonverbal, despite
normal peripheral hearing status [34].
Auditory Paracusias
These include auditory hallucinations (simple and
complex) and illusions. These need to be differ-
entiated from peduncular hallucinosis due to
pontine infarcts or other lesions that present with
very vivid, colorful, images. These are mostly
visual but can be auditory in nature with reports
of including people talking or shouting, when not
the reality [35–37].
Disorders of Time Perception
The subjective impression that time may pass
with excessive speed or not at all [38, 39].
Amusia
This may include subtypes of receptive, expressive,
and lexical amusia (impairment in reading music [40].
Other Social Disorders Associated
with Temporal Lobe Pathology
(Discussed Under Frontal Network
Syndromes Chapter)
Frontotemporal lobe disorders.
Right—behavioral FTD syndrome
Left—semantic aphasia
Schizophrenia
Autism spectrum conditions
Involuntaryemotionalexpressiondisorder(IEED)
Other Social Disorders Associated
with Temporal Lobe Pathology
(Discussed Under Memory
Syndromes Chapter)
Social impairment associated with memory
impairments
Urbach–Wiethe disease
Autoimmune encephalitis
Acquired prosopagnosia
Progressive prosopagnosia
Prosopagnosia secondary to stroke
The left temporal pole is implicated in the pro-
cessing and storage of proper names, important
for social interaction. This relationship is sup-
ported by both lesion studies and functional
imaging activation reports [41].
5 Temporal Lobe Syndromes](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-8-320.jpg)
![91
Traumatic Brain Injury (TBI):
Orbitofrontal,Anterior Temporal
Lobe and Uncinate Fasciculus
Predilection of Injury
Although brain injury may be both focal and dif-
fuse, extensive and widespread processes in both
a temporal domain and a spatial domain have
been established. In brief vascular, neurotrans-
mitter perturbation, glucose metabolism, and net-
work disturbances have been established. In
addition there is a more focal frontotemporal pre-
dilection of involvement in TBI. Both blast injury
and nonpenetrating head trauma of the anterior
temporal lobes, the inferior frontal lobes, and the
uncinate fasciculus as a functional unit appear to
bear the brunt of injury. This had been estab-
lished as long ago as 1937 and more recently cor-
roborated by MRI imaging data in 40 patients
[42]. Subsequent functional imaging with PET
scans has also implicated the inferior frontal and
temporal lobes indicating post-acute to chronic
injury hypermetabolism [43]. Finally, resting-
state network imaging has elucidated the much
more diffuse consequences of TBI revealing the
extensive cognitive and neuropsychiatric effects
of the disconnected hub networks [44].
Williams Syndrome
This syndrome presents during childhood with
features of:
Wide mouths
Upturned noses
Small chins
Curious starry eyes
Cardiac abnormalities
Hypercalcemia
Cognitively these children have intellectual
prowess in some areas and weakness in others.
They are described as being hypermusical, hyper-
narrative, and hypersocial. Notably they are
remarkably social displaying effervescence;
readily acquaint strangers are loquacious and
seem to delight in story telling. Some of their
weaknesses are akin to the autism spectrum
people. Certain neuroimaging features have been
reported including relatively smaller occipital
and parietal cortices and larger temporal lobes.
Functional imaging in relation to music has
revealed increased activation in the cerebellum,
temporal lobes, and amygdala [45].
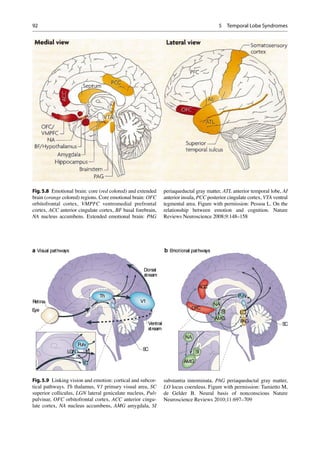
Emotion Disorders
Although the brain’s emotional circuitry is exten-
sive and widespread involving many cortical and
subcortical regions, the temporal lobe serves as
an important hub. Our understanding of the much
more expansive neural circuitry involved has
prompted a reappraisal of the critical, necessary,
and other involved brain regions in emotional
assessment, regulation, and impairments secondary
to disease processes (Fig. 5.8).
Evolutionary Insights
The brain has a two-tier system to help cope with
survival. Primates and humans inherited vision
as the predominant sense which is closely linked
to the emotional brain centers. These enable a
rapid and nonconscious reaction to environmen-
tal stimuli or predators that can be life saving,
being a more ancient and “unconscious,” system
which developed earlier in evolution concerned
with more elementary functions and survival,
not the least of which is dealing with the vast
sensory information that requires processing.
This is a standard infrastructure amongst verte-
brates in general. The conscious component
became a later elaboration of the primate brain
(Fig. 5.9) [46]. The occipitotemporal fasciculus
is a particularly large fiber tract in humans, part
of the inferior longitudinal fasciculus that allows
rapid transfer of visual information to the ante-
rior temporal lobe and amygdaloid complex for
environmental threat or predator evaluation and
human social and emotional salience processing
(Fig. 5.10) [47].
As humans we are particularly cooperative as
a species and our coordinated behavior is in
Emotion Disorders](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-9-320.jpg)
![93
marked contrast to all other animals [48].
Metacognition, a function of the frontopolar
cortex (BA 10), represents the apex of higher
cortical function ability. This region may be
divided into medial lateral and anterior regions
within BA 10, mediating self-reflection, intro-
spection, the monitoring and processing of
internal states with the externally acquired infor-
mation, episodic memory subfunctions such as
source memory and prospective memory, and
contextual and retrieval verification (Fig. 5.11)
[49, 50].
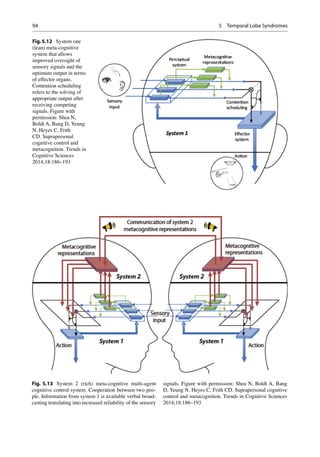
Shea et al. have proposed a two-level meta-
cognitive control system, a system 1 and system
2. System 1 (also called cognitively lean system)
of metacognition operates nonconsciously or
implicitly and is hypothesized to be involved in
processing information from the senses for intra-
personal cognition relevant to many animals
(Fig. 5.12). System 2 (also termed cognitively
rich system) is thought to be unique to humans
and is concerned with processing high-level
information amongst several conspecifics or peo-
ple, also termed suprapersonal control (Fig. 5.13).
Shea et al. conjecture that the suprapersonal
coordination metacognitive system antedated
system 1 or that which controlled the intraper-
sonal cognitive processes that underlie emotional
intelligence and inhibitory control [51]. In line
with these hypotheses is that one of the core frontal
functions of disinhibition was a critical development
in human evolution.
Fig.5.10 Occipito-
temporal pathway.
Figure with permission:
Catani M. Jones DK,
Donato R, ffytche
DH. Occipito-temporal
connections in the
human brain. Brain
2003;126:2093–2107
MNT
MLT
EP
EP
Fig. 5.11 Frontopolar cortex subregion activation pat-
terns (regional cerebral blood flow and blood oxygen level
dependent). MLT multitasking, MNT mentalizing, EP epi-
sodic memory. Figure adapted from: Gilbert SJ, Spengler
S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess
PW. Functional Specialization within the Rostral
Prefrontal Cortex (Area 10): A Meta-analysis. Journal of
Cognitive Neuroscience 2006;18:932–948
Emotion Disorders](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-11-320.jpg)
![95
Clinical
Emotional intelligence (EI) defined differently
according to the various clinical brain specialties
such as psychology, neurology, and psychiatry. EI
has been shown to be important for personal suc-
cess and career success and in navigating the social
complexities in day-to-day living [52]. EI impair-
ment may be apparent in both apparently healthy
people and after brain illnesses such as stroke, fron-
totemporal lobe dementia, Alzheimer’s disease,
and multiple sclerosis [53, 54]. Baron for example
has proposed that EI comprises of the group of
abilities that allow one to
1. Understand one’s own emotions and be able
to express feelings
2. Understand how others that one interacts with
feel and how one relates to them
3. Manage and be in control of one’s own
emotions
4. Use one’s emotions in adapting to one’s
environment
5. Generate positive emotions and use them for
self-motivation in facing challenges
The neural circuitry for EI involves orbitofron-
tal, anterior cingulate, the insula, and amygdaloid
complex regions [55, 56]. Processes that injure
these areas in particular include traumatic brain
injury, stroke, multiple sclerosis, and frontotempo-
ral lobe disorders. A holistic clinical brain injury
assessment should therefore involve all the clinical
brain specialties, neurological, neuropsychiatric,
psychological, and speech and language. EI assess-
ment has already been extensively embraced in the
corporate arena in view of its relationship with not
only career success but also productivity and insti-
tutional health [57, 58] and EI is amenable for
behavioral intervention programs [59, 60]. In a
study involving stroke patients, many different
brain lesions affected EI scores, including with
frontal, temporal, subcortical, and subtentorial
stroke lesions. The most significant abnormalities
were however associated with the frontal and tem-
poral region lesions, supporting Pessoa’s extended
emotional brain circuitry [61, 62].
Involuntary Emotional Expression
Disorder
Involuntary emotional expression disorder
(IEED) refers to clinically recognized syn-
dromes that are characterized by intermittent
inappropriate laughing and/or crying as well as
intermittent emotional lability. Lesion studies
have implicated the frontal primary motor (BA
4), premotor (BA 6), supplementary motor (BA
8), dorsal anterior cingulate gyrus, posterior
insular and parietal regions, and corticopontine
projections to the amygdala, hypothalamus,
and periaqueductal gray matter. In addition sei-
zure activity of these circuits may result in
laughing (gelastic) or crying episodes (dys-
crastic) [63]. Dextromethorphan is a sigma-1
receptor agonist and noncompetitive N-methyl-
d-aspartate (NMDA) receptor antagonist.
Quinidine is a competitive inhibitor of CYP2D6
which increases plasma levels of dextrometho-
rphan. In placebo, double-blinded studies
using validated IEED scales, the Center for
Neurologic Study-Lability Scale, in multiple
sclerosis and amyotrophic lateral sclerosis
patients, the combination drug was superior to
placebo [64].
References
1. Schumann C, Amaral DG. Stereological estimation
of the number of neurons in the human amygdaloid
complex. J Comp Neurol. 2005;491:320–9.
2. Bastir M, Rosas A, Gunz P, Peña-Melian A, Manzi G,
Harvati K, et al. Evolution of the base of the brain in
highly encephalized human species. Nat Commun.
2011;2:588. doi:10.1038/ncomms1593.
3. Semendeferi K, Barger N, Schenker N. Brain reorgani-
zation in humans and apes. The human brain evolving.
Gosport, IN: Stone Age Institute Press; 2010.
4. Barton RA, Aggleton JP, Grenyer R. Evolutionary
coherence of the mammalian amygdala. Proc Biol
Sci. 2003;270:539–43.
5. Dunbar RIM. The social brain: mind, language and
society in evolutionary perspective. Ann Rev
Anthropol. 2003;32:163–81.
6. Pelphrey KA, Viola RJ, McCarthy G. When strang-
ers pass: processing of mutual and averted social
gaze in the superior temporal sulcus. Psychol Sci.
2004;15:598–603.
References](https://image.slidesharecdn.com/temporallobesyndrome-220709151117-1d3c0312/85/temporal-lobe-syndrome-pdf-13-320.jpg)

