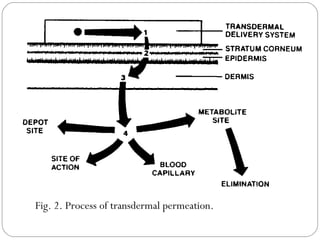
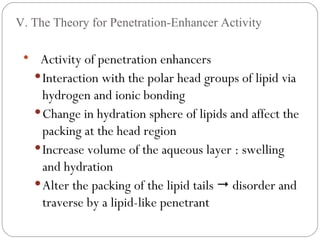
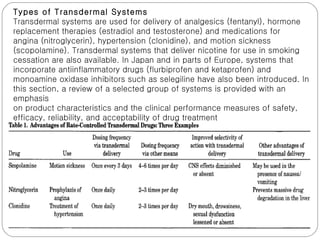
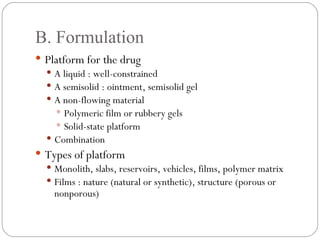
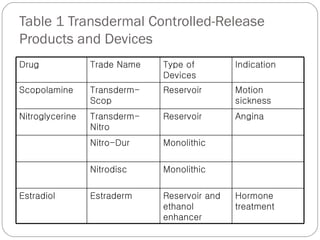
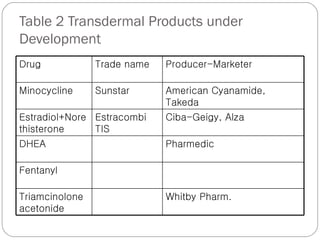
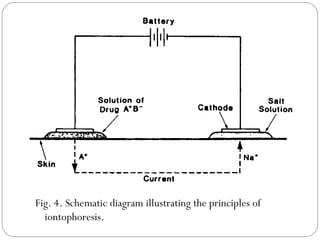
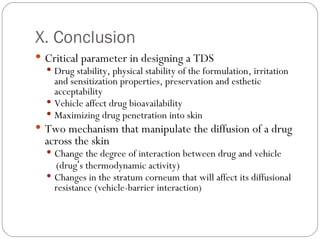
Transdermal drug delivery systems provide several advantages over oral drug administration, including avoidance of first-pass metabolism, controlled drug levels over time, and increased patient compliance. The structure of the skin, particularly the stratum corneum layer, influences drug permeation rates. Optimization of drug formulations includes the use of penetration enhancers to increase the drug's ability to partition into the skin. Examples of drugs delivered via transdermal patches include scopolamine for motion sickness, nitroglycerin for angina, and estradiol for hormone therapy.