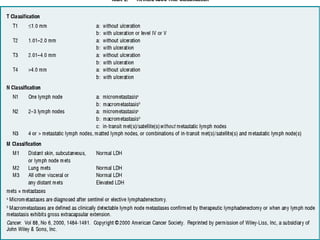
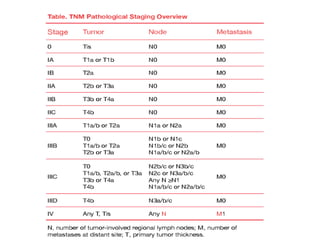
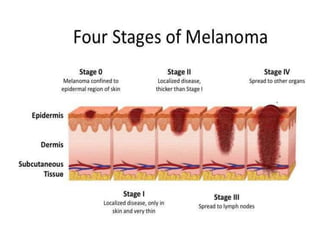
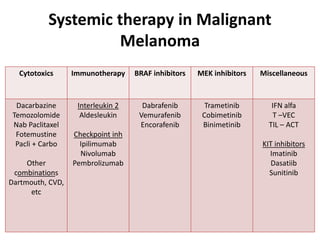
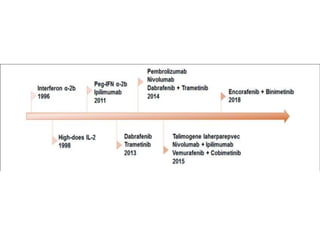
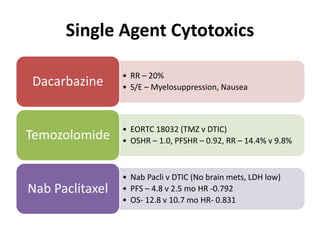
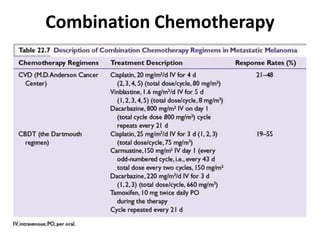
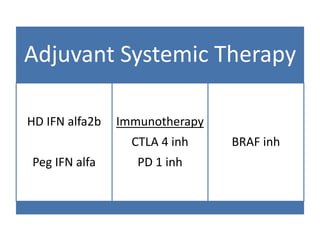
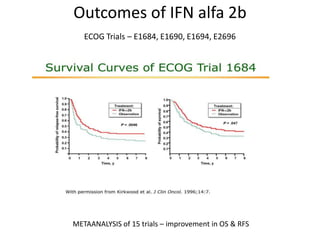
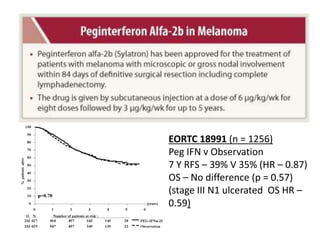
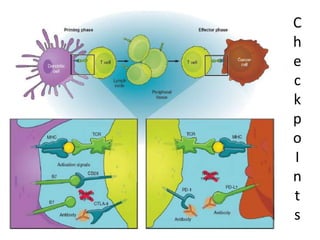
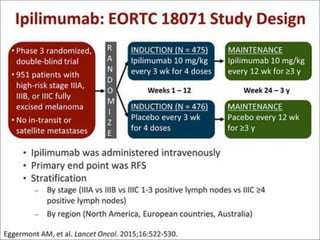
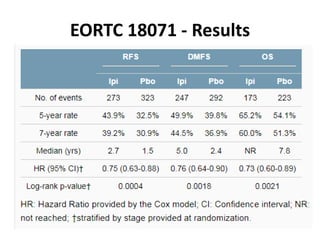
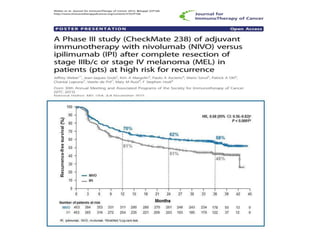
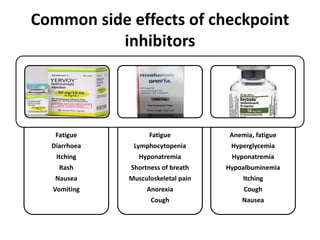
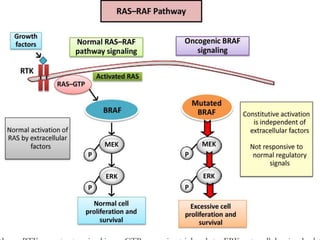
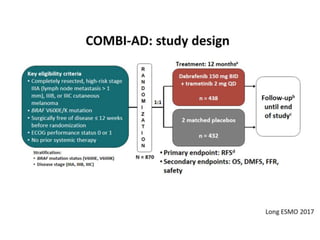
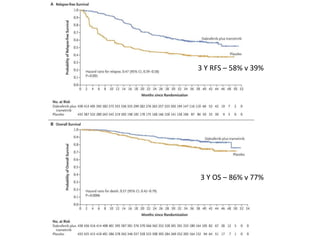
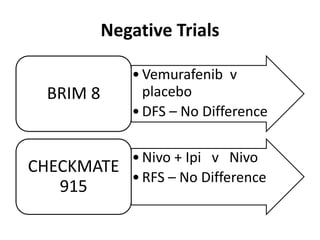
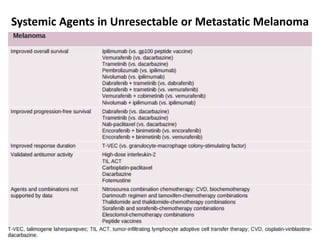
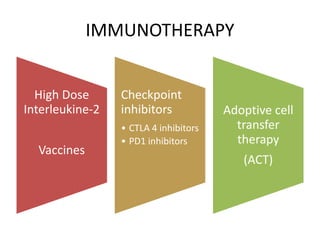
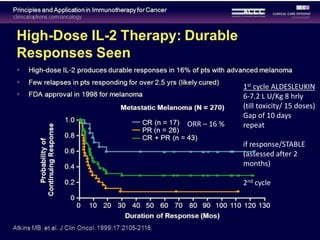
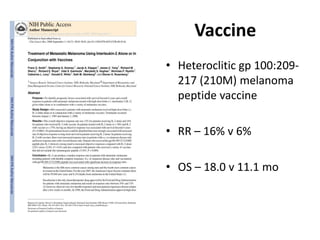
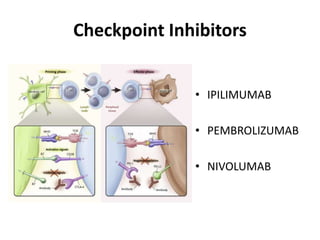
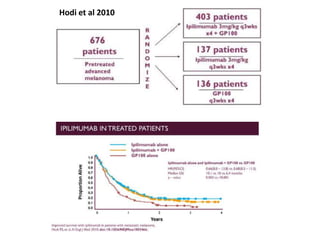
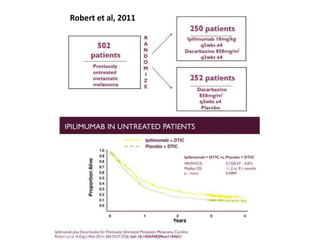
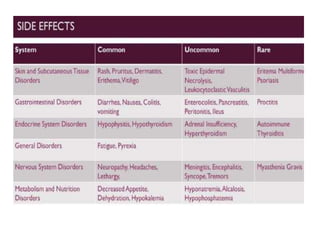
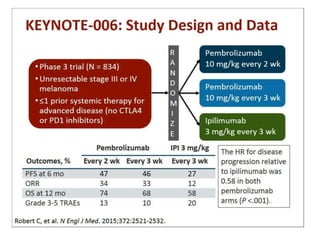
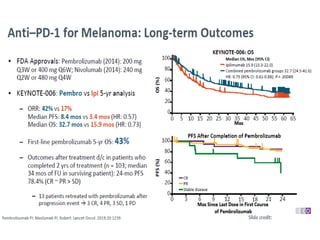
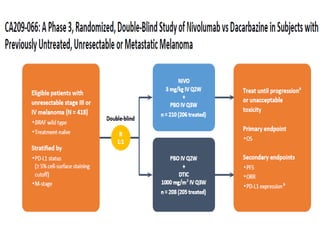
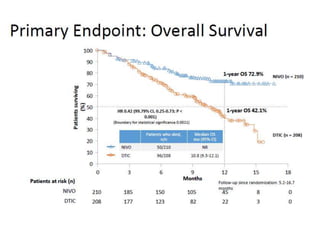
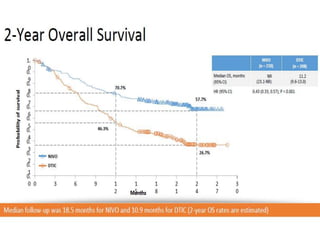
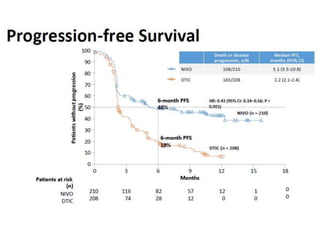
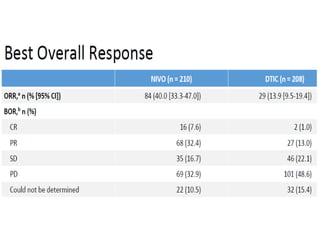
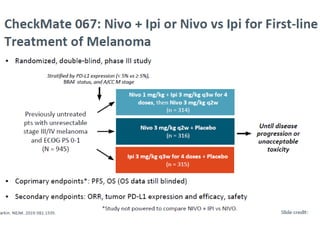
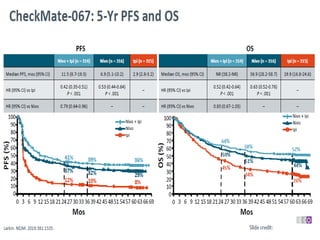
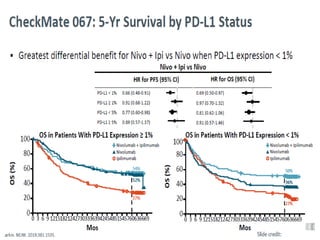
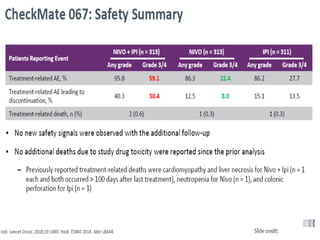
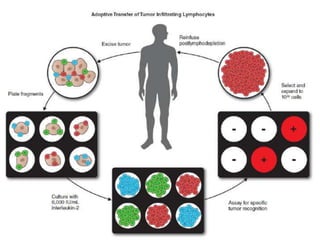
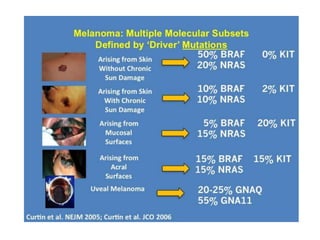
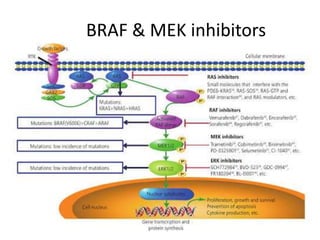
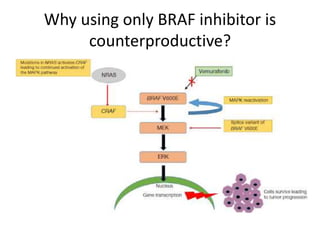
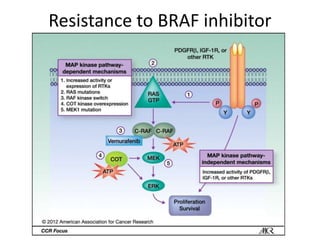
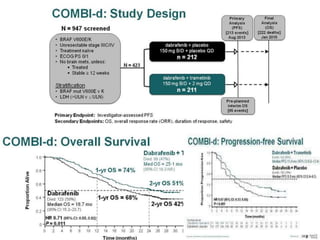
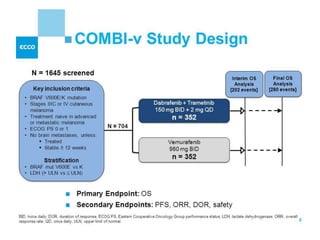
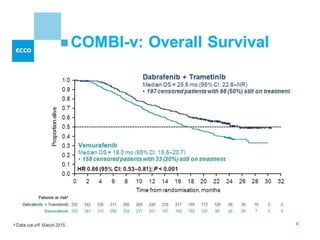
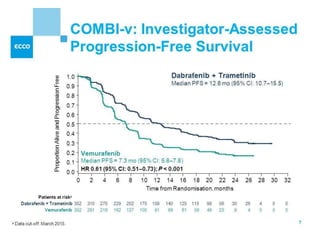
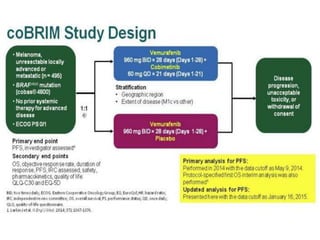
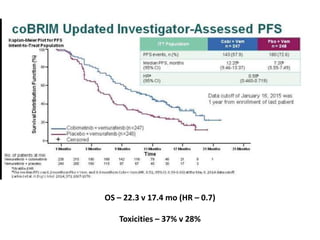
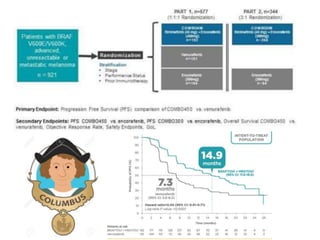
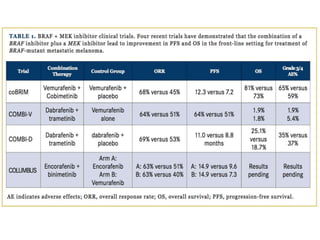
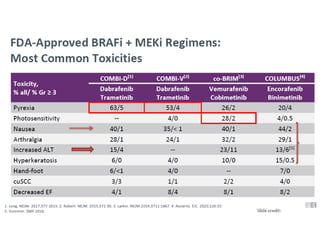
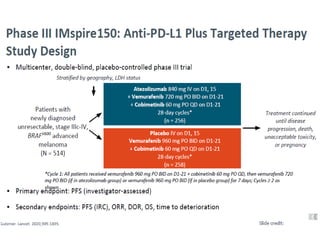
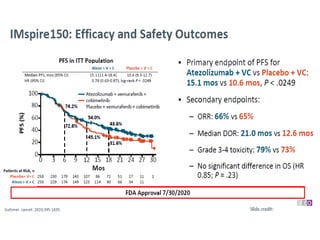
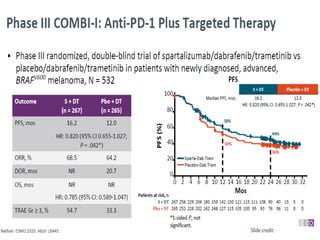
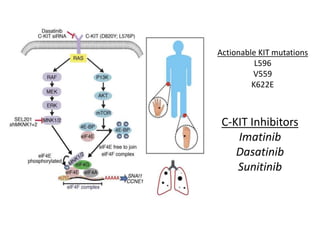
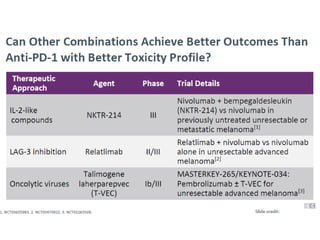
This document summarizes systemic therapy options for malignant melanoma, including cytotoxics, immunotherapy, targeted therapies, and their outcomes. Key points discussed are: (1) Immunotherapy with checkpoint inhibitors like ipilimumab and pembrolizumab have significantly improved response rates and survival compared to traditional chemotherapies; (2) Combining BRAF and MEK inhibitors has become an important targeted therapy approach as it improves outcomes over BRAF inhibitors alone by delaying resistance; (3) Emerging evidence suggests combining immunotherapy and targeted therapies may provide further benefit.