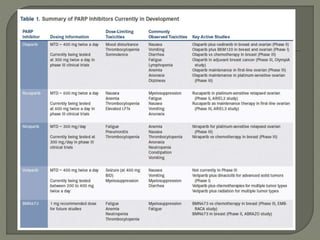
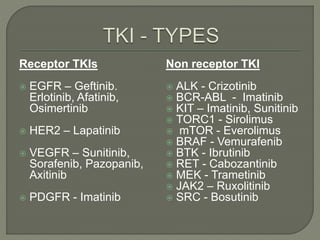
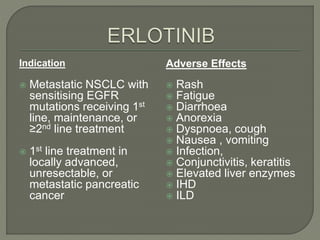
The document discusses various targeted therapies used in oncology including monoclonal antibodies, small molecule inhibitors, and tyrosine kinase inhibitors. It provides information on drug indications, mechanisms of action, FDA approvals, and common adverse effects for several targeted agents such as palbociclib, bortezomib, imatinib, crizotinib, ceritinib, osimertinib, and vemurafenib. The document is intended to educate medical practitioners on modern targeted therapies for cancer treatment.